| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Chlorobuta-1,3-diene | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 741875 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.381 | ||
| EC Number |
| ||
| 277888 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1991 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H5Cl | |||
| Molar mass | 88.53 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Pungent, ether-like | ||
| Density | 0.9598 g/cm3 | ||
| Melting point | −130 °C (−202 °F; 143 K)[1] | ||
| Boiling point | 59.4 °C (138.9 °F; 332.5 K)[1] | ||
| 0.026 g/100 mL | |||
| Solubility in diethyl ether | miscible[1] | ||
| Solubility in acetone | miscible[1] | ||
| Solubility in benzene | miscible[1] | ||
| Solubility in ethanol | soluble | ||
| Vapor pressure |
| ||
Refractive index (nD)
|
1.4583 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Highly flammable, irritant, toxic | ||
| GHS labelling:[1] | |||
  
| |||
| Danger | |||
| H225, H302, H315, H319, H332, H335, H350, H373 | |||
| P203, P210, P233, P240, P241, P242, P243, P260, P261, P264, P264+P265, P270, P271, P280, P281, P301+P317, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P317, P318, P319, P321, P330, P332+P317, P337+P313, P362+P364, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −4 °C (25 °F; 269 K)[1] | ||
| Explosive limits | 1.9%–20.0%[1] | ||
Threshold limit value (TLV)
|
10 ppm (skin)[1] (TWA) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
450 mg/kg (rat, oral) | ||
LC50 (median concentration)
|
3207 ppm (rat, 4 hr)[2] | ||
LCLo (lowest published)
|
| ||
| NIOSH (US health exposure limits):[4] | |||
PEL (Permissible)
|
25 ppm (90 mg/m3, TWA, skin) | ||
REL (Recommended)
|
1 ppm (3.6 mg/m3, Ca C, [15-minute]) | ||
IDLH (Immediate danger)
|
300 ppm (1086 mg/m3) | ||
| Related compounds | |||
Related Dienes
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chloroprene (IUPAC name 2-chlorobuta-1,3-diene) is a chemical compound with the molecular formula CH2=CCl−CH=CH2.[5] Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene, better known as neoprene, a type of synthetic rubber.
History
[edit]Although it may have been discovered earlier, chloroprene was largely developed by DuPont during the early 1930s, specifically with the formation of neoprene in mind.[6] The chemists Elmer K. Bolton, Wallace Carothers, Arnold Collins and Ira Williams are generally accredited with its development and commercialisation although the work was based upon that of Julius Arthur Nieuwland, with whom they collaborated.[7]
Production
[edit]Chloroprene is produced in three steps from 1,3-butadiene: (i) chlorination, (ii) isomerization of part of the product stream, and (iii) dehydrochlorination of 3,4-dichlorobut-1-ene.
Chlorine adds to 1,3-butadiene to afford a mixture of 3,4-dichlorobut-1-ene and 1,4-dichlorobut-2-ene. The 1,4-dichloro isomer is subsequently isomerized to 3,4 isomer, which in turn is treated with base to induce dehydrochlorination to 2-chlorobuta-1,3-diene. This dehydrohalogenation entails loss of a hydrogen atom in the 3 position and the chlorine atom in the 4 position thereby forming a double bond between carbons 3 and 4:[5]
- CH2=CHCH=CH2 + Cl2 → ClCH2CH=CHCH2Cl
- ClCH2CH=CHCH2Cl → ClCH2CHClCH=CH2
- ClCH2CHClCH=CH2 → CH2=CClCH=CH2 + HCl
The chief impurity in chloroprene prepared in this way is 1-chlorobuta-1,3-diene, which is usually separated by distillation.[5] In 1983, approximately 2 million kilograms (2,200 short tons) were produced in this manner.
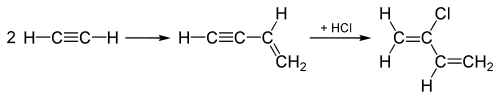
Acetylene process
[edit]Until the 1960s, chloroprene production was dominated by the "acetylene process," which was modeled after the original synthesis of vinylacetylene.[6] In this process, acetylene is dimerized to give vinyl acetylene, which is then combined with hydrogen chloride to afford 4-chloro-1,2-butadiene (an allene derivative), which in the presence of copper(I) chloride, rearranges to the targeted 2-chlorobuta-1,3-diene:[5]
The acetylene process is energy-intensive and has high investment costs. Furthermore, the intermediate vinyl acetylene is unstable. This "acetylene process" has been replaced by the chlorination of 1,3-butadiene.
Hazards
[edit]Chloroprene may explosively polymerize. The peroxide catalyzes this.[8]
Occupational exposure limits
[edit]A table of occupational exposure limits (OELs) from various jurisdictions follows. In general, the OELs range from 0.55 to 25 ppm.[9]
| Occupational exposure limits for chloroprene[citation needed] | |
|---|---|
| Organization | Concentration |
| NIOSH REL | 1 ppm |
| ACGIH TLV 8-hour TWA | 10 ppm |
| OSHA PEL 8-hour TWA | 25 ppm |
| Mine Safety and Health Administration | 25 ppm |
| Austria OEL MAK-TMW | 5 ppm |
| Belgium OEL TWA | 10 ppm |
| Denmark OEL ceiling concentration | 1 ppm |
| Finland OEL TWA | 1 ppm |
| France OEL VME | 10 ppm |
| Hungary OEL TWA | 5 ppm |
| Iceland OEL Short Term Exposure Limit (STEL) | 1 ppm |
| Korea OEL TWA | 10 ppm |
| Mexico OEL TWA | 10 ppm |
| New Zealand OEL TWA | 10 ppm |
| Norway OEL TWA | 1 ppm |
| Peru OEL TWA | 10 ppm |
| Poland OEL MAC TWA | 0.55 ppm |
| Sweden OEL TWA | 1 ppm |
| Switzerland OEL MAK-week | 5 ppm |
| The Netherlands OEL MAC-TGG | 5 ppm |
Environment
[edit]The fate of chloroprene in the environment has been examined.[10] Due to its volatility and extreme reactivity, it is not expected to bioaccumulate.[11]
Health
[edit]Chloroprene is toxic. Chloroprene is potentially carcinogenic, can cause temporary hair loss on the exposed area, and can cause damage to the eyes and skin.[12]
The Environmental Protection Agency designated chloroprene as likely to be carcinogenic to humans based on evidence from studies that showed a statistically significant association between occupational chloroprene exposure and the risk of lung cancer.[11] There are criticisms of this report that indicate that the unsafe exposure levels may have been exaggerated badly based on levels for known carcinogens.[13]
Chronic exposure to chloroprene may have the following symptoms: liver function abnormalities, disorders of the cardiovascular system, and depression of the immune system.[1]
One fatality as a result of chloroprene intoxication has been recorded, which was a result of cleaning a container used for chloroprene.[14]
References
[edit]- ^ a b c d e f g h i j k "Chloroprene - Laboratory Chemical Safety Summary (LCSS)". pubchem.ncbi.nlm.nih.gov. National Library of Medicine. Retrieved 25 December 2025.
- ^ a b "ß-Chloroprene". Immediately Dangerous to Life or Health Concentrations. National Institute for Occupational Safety and Health.
- ^ "Chloroprene, Stabilized | Cameo Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2018-12-14.
- ^ "NIOSH Pocket Guide to Chemical Hazards".
- ^ a b c d Rossberg, M.; Lendle, W.; Pfleiderer, G.; Tögel, A.; Dreher, E.-L.; Langer, E.; Rassaerts, H.; Kleinschmidt, P.; Strack, H.; Cook, R.; Beck, U.; Lipper, K.-A.; Torkelson, T. R.; Löser, E.; Beutel, K.K.; Mann, T. (15 July 2006). "Chlorinated Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_233.pub2. ISBN 978-3-527-30673-2.
- ^ a b Carothers, Wallace H.; Williams, Ira.; Collins, Arnold M.; Kirby, James E. (November 1931). "Acetylene Polymers and their Derivatives. II. A New Synthetic Rubber: Chloroprene and its Polymers". Journal of the American Chemical Society. 53 (11): 4203–4225. doi:10.1021/ja01362a042.
- ^ Smith, John K. (January 1985). "The Ten-Year Invention: Neoprene and Du Pont Research, 1930-1939". Technology and Culture. 26 (1): 34–55. doi:10.2307/3104528. JSTOR 3104528. S2CID 113234844.
- ^ "Chloroprene, Stabilized | CAMEO Chemicals". cameochemicals.noaa.gov. NOAA. Retrieved 25 December 2025.
- ^ "Template Package 4". Centers for Disease Control and Prevention. Retrieved 2018-11-24.
- ^ National Institute of Environmental Health Sciences. National Toxicology Program (2016). Report on Carcinogens, fourteenth edition. ISBN 978-1-5231-0852-7. OCLC 990561140.
- ^ a b "4.7 - Evaluation of Carcinogenicity". Toxicological Review of Chloroprene - In Support of Summary Information on the Integrated Risk Information System (IRIS) (Report). Washington, DC: U.S. Environmental Protection Agency. 2010. p. 96. EPA/635/R-09/010F.
- ^ "Hazardous Substance Fact Sheet" (PDF). New Jersey Department of Health and Senior Services.
- ^ "The IRIS Review Process: Chloroprene and the Criticality of Good Science" (PDF). House Committee on Science, Space, and Technology Republicans. S2CID 33811055.
- ^ Rickert, Annette; Hartung, Benno; Kardel, Bernd; Teloh, Johanna; Daldrup, Thomas (2012-02-10). "A fatal intoxication by chloroprene". Forensic Science International. 215 (1–3): 110–113. doi:10.1016/j.forsciint.2011.03.029. ISSN 0379-0738. PMID 21511420.
External links
[edit]- IARC Monograph "Chloroprene."