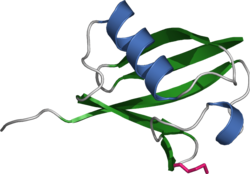
RING finger protein 4 (RNF4) / Small nuclear ring finger protein (SNURF) is a 190-amino acid protein that in humans is encoded by the RNF4 gene [5][6]
The RNF4 gene contains 2 nuclear localization signals (NLS), 8 exons, and is approximately 47 kb in length, and weighs ~18 kDa (without isoforms or tags)[7][8][9]
The protein encoded by this gene contains a RING finger domain and acts as a transcription factor. This protein has been shown to interact with, and inhibit the activity of, TRPS1,[10] a transcription suppressor of GATA-mediated transcription. This allows TRPS1-regulated genes to be expressed more. Transcription repressor ZNF278/PATZ1 is found to interact with this protein, and thus reduce the enhancement of androgen receptor-dependent transcription mediated by this protein. Studies of the mouse and rat counterparts suggested a role of this protein in spermatogenesis.[6]
Structure
[edit]RNF4 protein consists of a dimeric RING domain. The combination of two RING domains is what allows for efficient post translational modification of ubiquitination [11] (the RNF4 tags proteins with ubiquitin chains for degradation to be done by the Proteasome). RNF4 also contains four N-terminal SIM repeats (SmallUbiquitinMOdifier-interacting motifs), as well as one C-terminal which act as mediators when their selective binding to a polysumolated substrate takes place. [9][12]
RNF4's N-terminal SIM cluster, binds to poly-SUMO chains or units, confirming specificity and as a result RNF4 targets SUMOylated proteins for ubiquination. [13]
Functions
[edit]RNF4 functions as a STUbL (SUMO-targeted ubiquitin ligase). This is an E3 ubiquitin ligase that recognized proteins that have been modified with SUMO groups/chains. Specifically, during ubiquitination, the SUMOylated proteins are tagged witih polyubiquitin chains. These chains are recognized by the proteasome, which then unfolds the protein to degrade it in to peptides.
RNF4 also contributes to genome stability. It can do this through the repair of damaged DNA prior to mitosis. SUMOylated DNA-protein crosslinks (DPCs) that threaten the stability of a genome are removed by RNF4. After DNA replication, RNF4 protein helps prevent chromosome segregation defects and apoptosis through targeted SUMOylation.[14] Since this occurs outside of DNA replication, RNF4 is able to protect the cell even outside of the S phase of the cell cycle. The specific mechanisms on how are still being studied.
RNF4 has derepressing abilities/ gene regulating abilities, as mentioned in Wang's study from 2014. RNF4 derepresses gene expression by targeting the proteins that bind to methylated DNA. After this they are marked for ubiquitination[15]
A recent study looked into what role RNF4 would take on with under-replicated DNA. Taking place at common fragile sites (CFSs), the under-replicated DNA that continued on after S phase were packed into 53BP1-NuclearBodies by cells to protect them from damage. These nuclear bodies allow the DNA to continue through the cell cycle. This study used cells missing the RNF4 gene and saw there was an increase in the nuclear bodies. This supported their idea that RNF4 regulates protein degradation, and if not present there would be under-replication.[16]
The overexpression of RNF4 resulted in an increase in the enzymatic activities of endogenous base excision repair enzymes TDG and APE1 as well an increase in the mismatch repair efficiency. This reinforces RNF4s role in DNA repair.[8]
Another study involving mice showed the RNF4 protein is essential for embryonic development. Without this gene, embryo growth comes to a halt. Additionally, in rats, acute promyelocytic leukemia (APL) is treated with arsenic since it induces conjugation of SUMO and degrades PML (promyelocytic leukaemia) protein.[17] RNF4 binds to poly-SUMO2-modified PML through the four N-terminal SIMs previously mentioned and the absence of RNF4 causes there to be no degradation of PML protein by arsenic.
There are currently no human diseases associated with this protein. Studies indicate that RNF4 is involved in regulating degradation, genome protection and stability from replication and DPCs, which highlights its critical role in normal cellular function.[14][16]
Interactions
[edit]RNF4 has been shown to interact with TCF20,[18] PATZ1, [19][20] TRPS1[10] and Androgen receptor.[20][21][22] RNF4 has been shown to be responsible for the degradation of the Werner syndrome helicase in MSI-H cells after WRN inhibition.[23] Studies show RNF4 binds to both ubiquitin charged UbcH5a (strongly) and uncharged UbcH5a (weakly). The RNF4 dimers activate the E2 ubiquitin thioester for catalysis.[24]
See also
[edit]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000063978 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000029110 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Chiariotti L, Benvenuto G, Fedele M, Santoro M, Simeone A, Fusco A, et al. (January 1998). "Identification and characterization of a novel RING-finger gene (RNF4) mapping at 4p16.3". Genomics. 47 (2): 258–265. doi:10.1006/geno.1997.5105. PMID 9479498.
- ^ a b "Entrez Gene: RNF4 ring finger protein 4".
- ^ Chiariotti L, Benvenuto G, Fedele M, Santoro M, Simeone A, Fusco A, et al. (January 1998). "Identification and characterization of a novel RING-finger gene (RNF4) mapping at 4p16.3". Genomics. 47 (2): 258–265. doi:10.1006/geno.1997.5105. PMID 9479498.
- ^ a b "Entry - *602850 - Ring Finger Protein 4; RNF4". omim.org. Retrieved 2025-12-04.
- ^ a b "UniProt". UniProt. Retrieved 2025-12-04.
- ^ a b Kaiser FJ, Möröy T, Chang GT, Horsthemke B, Lüdecke HJ (October 2003). "The RING finger protein RNF4, a co-regulator of transcription, interacts with the TRPS1 transcription factor". The Journal of Biological Chemistry. 278 (40): 38780–38785. doi:10.1074/jbc.M306259200. PMID 12885770.
- ^ Plechanovová A, Jaffray EG, McMahon SA, Johnson KA, Navrátilová I, Naismith JH, et al. (August 2011). "Mechanism of ubiquitylation by dimeric RING ligase RNF4". Nature Structural & Molecular Biology. 18 (9): 1052–1059. doi:10.1038/nsmb.2108. PMC 3326525. PMID 21857666.
- ^ Kung CC, Naik MT, Wang SH, Shih HM, Chang CC, Lin LY, et al. (August 2014). "Structural analysis of poly-SUMO chain recognition by the RNF4-SIMs domain". The Biochemical Journal. 462 (1): 53–65. doi:10.1042/BJ20140521. PMID 24844634.
- ^ Kung CC, Naik MT, Wang SH, Shih HM, Chang CC, Lin LY, et al. (August 2014). "Structural analysis of poly-SUMO chain recognition by the RNF4-SIMs domain". The Biochemical Journal. 462 (1): 53–65. doi:10.1042/BJ20140521. PMID 24844634.
- ^ a b Liu JC, Kühbacher U, Larsen NB, Borgermann N, Garvanska DH, Hendriks IA, et al. (September 2021). "Mechanism and function of DNA replication-independent DNA-protein crosslink repair via the SUMO-RNF4 pathway". The EMBO Journal. 40 (18) e107413. doi:10.15252/embj.2020107413. PMC 8441304. PMID 34346517.
- ^ Wang Y (December 2014). "RING finger protein 4 (RNF4) derepresses gene expression from DNA methylation". The Journal of Biological Chemistry. 289 (49): 33808–33813. doi:10.1074/jbc.C114.611558. PMC 4256315. PMID 25355316.
- ^ a b Oram MK, Baxley RM, Simon EM, Lin K, Chang YC, Wang L, et al. (March 2024). "RNF4 prevents genomic instability caused by chronic DNA under-replication". DNA Repair. 135 103646. doi:10.1016/j.dnarep.2024.103646. PMC 10948022. PMID 38340377.
- ^ Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, et al. (May 2008). "RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation". Nature Cell Biology. 10 (5): 538–546. Bibcode:2008NaCB...10..538T. doi:10.1038/ncb1716. PMID 18408734.
- ^ Lyngsø C, Bouteiller G, Damgaard CK, Ryom D, Sanchez-Muñoz S, Nørby PL, et al. (August 2000). "Interaction between the transcription factor SPBP and the positive cofactor RNF4. An interplay between protein binding zinc fingers". The Journal of Biological Chemistry. 275 (34): 26144–26149. doi:10.1074/jbc.M003405200. PMID 10849425.
- ^ Fedele M, Benvenuto G, Pero R, Majello B, Battista S, Lembo F, et al. (March 2000). "A novel member of the BTB/POZ family, PATZ, associates with the RNF4 RING finger protein and acts as a transcriptional repressor". The Journal of Biological Chemistry. 275 (11): 7894–7901. doi:10.1074/jbc.275.11.7894. PMID 10713105.
- ^ a b Pero R, Lembo F, Palmieri EA, Vitiello C, Fedele M, Fusco A, et al. (February 2002). "PATZ attenuates the RNF4-mediated enhancement of androgen receptor-dependent transcription". The Journal of Biological Chemistry. 277 (5): 3280–3285. doi:10.1074/jbc.M109491200. PMID 11719514.
- ^ Moilanen AM, Poukka H, Karvonen U, Häkli M, Jänne OA, Palvimo JJ (September 1998). "Identification of a novel RING finger protein as a coregulator in steroid receptor-mediated gene transcription". Molecular and Cellular Biology. 18 (9): 5128–5139. doi:10.1128/mcb.18.9.5128. PMC 109098. PMID 9710597.
- ^ Poukka H, Aarnisalo P, Santti H, Jänne OA, Palvimo JJ (January 2000). "Coregulator small nuclear RING finger protein (SNURF) enhances Sp1- and steroid receptor-mediated transcription by different mechanisms". The Journal of Biological Chemistry. 275 (1): 571–579. doi:10.1074/jbc.275.1.571. PMID 10617653.
- ^ Rodríguez Pérez F, Natwick D, Schiff L, McSwiggen D, Heckert A, Huey M, et al. (July 2024). "WRN inhibition leads to its chromatin-associated degradation via the PIAS4-RNF4-p97/VCP axis". Nature Communications. 15 (1) 6059. Bibcode:2024NatCo..15.6059R. doi:10.1038/s41467-024-50178-3. PMC 11258360. PMID 39025847.
- ^ Plechanovová A, Jaffray EG, McMahon SA, Johnson KA, Navrátilová I, Naismith JH, et al. (August 2011). "Mechanism of ubiquitylation by dimeric RING ligase RNF4". Nature Structural & Molecular Biology. 18 (9): 1052–1059. doi:10.1038/nsmb.2108. PMC 3326525. PMID 21857666.
Further reading
[edit]- Moilanen AM, Poukka H, Karvonen U, Häkli M, Jänne OA, Palvimo JJ (September 1998). "Identification of a novel RING finger protein as a coregulator in steroid receptor-mediated gene transcription". Molecular and Cellular Biology. 18 (9): 5128–5139. doi:10.1128/mcb.18.9.5128. PMC 109098. PMID 9710597.
- Hadano S, Ishida Y, Ikeda JE (June 1998). "The primary structure and genomic organization of five novel transcripts located close to the Huntington's disease gene on human chromosome 4p16.3". DNA Research. 5 (3): 177–186. doi:10.1093/dnares/5.3.177. PMID 9734812.
- Poukka H, Aarnisalo P, Santti H, Jänne OA, Palvimo JJ (January 2000). "Coregulator small nuclear RING finger protein (SNURF) enhances Sp1- and steroid receptor-mediated transcription by different mechanisms". The Journal of Biological Chemistry. 275 (1): 571–579. doi:10.1074/jbc.275.1.571. PMID 10617653.
- Fedele M, Benvenuto G, Pero R, Majello B, Battista S, Lembo F, et al. (March 2000). "A novel member of the BTB/POZ family, PATZ, associates with the RNF4 RING finger protein and acts as a transcriptional repressor". The Journal of Biological Chemistry. 275 (11): 7894–7901. doi:10.1074/jbc.275.11.7894. PMID 10713105.
- Galili N, Nayak S, Epstein JA, Buck CA (May 2000). "Rnf4, a RING protein expressed in the developing nervous and reproductive systems, interacts with Gscl, a gene within the DiGeorge critical region". Developmental Dynamics. 218 (1): 102–111. doi:10.1002/(SICI)1097-0177(200005)218:1<102::AID-DVDY9>3.0.CO;2-A. PMID 10822263.
- Lyngsø C, Bouteiller G, Damgaard CK, Ryom D, Sanchez-Muñoz S, Nørby PL, et al. (August 2000). "Interaction between the transcription factor SPBP and the positive cofactor RNF4. An interplay between protein binding zinc fingers". The Journal of Biological Chemistry. 275 (34): 26144–26149. doi:10.1074/jbc.M003405200. PMID 10849425.
- Häkli M, Karvonen U, Jänne OA, Palvimo JJ (June 2001). "The RING finger protein SNURF is a bifunctional protein possessing DNA binding activity". The Journal of Biological Chemistry. 276 (26): 23653–23660. doi:10.1074/jbc.M009891200. PMID 11319220.
- Saville B, Poukka H, Wormke M, Janne OA, Palvimo JJ, Stoner M, et al. (January 2002). "Cooperative coactivation of estrogen receptor alpha in ZR-75 human breast cancer cells by SNURF and TATA-binding protein". The Journal of Biological Chemistry. 277 (4): 2485–2497. doi:10.1074/jbc.M109021200. PMID 11696545.
- Pero R, Lembo F, Palmieri EA, Vitiello C, Fedele M, Fusco A, et al. (February 2002). "PATZ attenuates the RNF4-mediated enhancement of androgen receptor-dependent transcription". The Journal of Biological Chemistry. 277 (5): 3280–3285. doi:10.1074/jbc.M109491200. PMID 11719514.
- Yan W, Hirvonen-Santti SJ, Palvimo JJ, Toppari J, Jänne OA (October 2002). "Expression of the nuclear RING finger protein SNURF/RNF4 during rat testis development suggests a role in spermatid maturation". Mechanisms of Development. 118 (1–2): 247–253. doi:10.1016/S0925-4773(02)00261-7. PMID 12351196. S2CID 15579434.
- Pero R, Lembo F, Chieffi P, Del Pozzo G, Fedele M, Fusco A, et al. (September 2003). "Translational regulation of a novel testis-specific RNF4 transcript". Molecular Reproduction and Development. 66 (1): 1–7. doi:10.1002/mrd.10322. PMID 12874792. S2CID 6206480.
- Kaiser FJ, Möröy T, Chang GT, Horsthemke B, Lüdecke HJ (October 2003). "The RING finger protein RNF4, a co-regulator of transcription, interacts with the TRPS1 transcription factor". The Journal of Biological Chemistry. 278 (40): 38780–38785. doi:10.1074/jbc.M306259200. PMID 12885770.
- Lin DY, Fang HI, Ma AH, Huang YS, Pu YS, Jenster G, et al. (December 2004). "Negative modulation of androgen receptor transcriptional activity by Daxx". Molecular and Cellular Biology. 24 (24): 10529–10541. doi:10.1128/MCB.24.24.10529-10541.2004. PMC 533990. PMID 15572661.
External links
[edit]- RNF4+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)




