リチウム ビス (トリメチルシリル) アミド
| リチウム ビス (トリメチルシリル) アミド | |
|---|---|
 Monomer (does not exist)
| |
 Cyclic trimer
| |

| |
Lithium 1,1,1-trimethyl-N-(trimethylsilyl)silanaminide | |
別称 Lithium hexamethyldisilazide Hexamethyldisilazane lithium salt | |
| 識別情報 | |
| CAS登録番号 | 4039-32-1 |
| PubChem | 2733832 |
| ChemSpider | 21170111 |
| |
| 特性 | |
| 化学式 | C6H18LiNSi2 |
| モル質量 | 167.33 g mol−1 |
| 外観 | 白色固体 |
| 密度 | 0.86 g/cm3 at 25 °C |
| 融点 |
71 - 72°C |
| 沸点 |
80 - 80°C |
| 危険性 | |
| 主な危険性 | 可燃性、腐食性 |
| 特記なき場合、データは常温 (25 °C)・常圧 (100 kPa) におけるものである。 | |
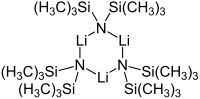
リチウム ビス (トリメチルシリル)アミド (Lithium bis(trimethylsilyl)amide) は、化学式 LiN(SiMe3)2 で表されるリチウム化された有機ケイ素化合物である。通常LiHMDS (lithium hexamethyldisilazide-共役酸であるHMDSへの参照) と省略される。主に強力な非求核塩基および配位子として使用される。多くのリチウム試薬と同様に、凝集する傾向があり、配位種がない場合は環状三量体を形成する。
調製
[編集]LiHMDSは市販品が入手できるが、ビス(トリメチルシリル)アミンをn-ブチルリチウムで脱プロトン化することによっても得られる[1]。この反応はin situで行うことができる[2]。
- HN(SiMe3)2 + C4H9Li → LiN(SiMe3)2 + C4H10
形成された化合物は昇華または蒸留で精製することができる。
反応と応用
[編集]塩基として
[編集]LiHMDSは、有機化学においてしばしば非求核塩基として用いられる[3]。その共役酸のpKaは約26であり[4]、リチウムジイソプロピルアミド (共役酸のpKa〜36)などの他のリチウム塩基よりも塩基性が低いが、立体障害が大きく、そのため求核性が低い。アセチリド[3]またはリチウムエノラートを含むさまざまな有機リチウム化合物の形成に使用できる[2]。

そのため、さまざまなカップリング反応、特にフレーター・ゼーバッハアルキル化や混合クライゼン縮合などの炭素-炭素結合形成反応で使用される。
四硫化四窒素の代替合成では、(Me3Si2N]2Sを あらかじめ形成されたSN結合の 前駆体として使用する。[Me3Si2N]2Sは、リチウム ビス(トリメチルシリル)アミドと二塩化硫黄 (SCl2)の反応によって調製される。
- 2 [(CH3)3Si]2NLi + SCl2 → [((CH3)3Si)2N]2S + 2 LiCl
[((CH3)3Si)2N]2Sは、SCl2と塩化スルフリル (SO2Cl2)を組み合わせたものと反応してS4N4、クロロトリメチルシラン、二酸化硫黄を形成する[5]。
- 2[((CH3)3Si)2N]2S + 2SCl2 + 2SO2Cl2 → S4N4 + 8 (CH3)3SiCl + 2SO2
配位子として
[編集]LiHMDSは、塩メタセシス反応によって広範囲の金属ハロゲン化物と反応して、金属ビス (トリメチルシリル) アミドを生成する。
- MXx + x Li(hmds) → M(hmds)x + x LiX
- (X = Cl, Br, I and sometimes F)
金属ビス (トリメチルシリル )アミド錯体は、配位子のために親油性であるため、非極性有機溶媒に溶解する。これにより、可溶化するのが難しい金属ハロゲン化物よりも反応性が高くなることがよくある。配位子のかさ高さのため、錯体はより離散的、単量体的になり、反応性がさらに高まる。塩基が組み込まれているこれらの化合物は、プロトン性配位子前駆体と便利に反応して他の金属錯体を生成するため、より複雑な配位化合物の重要な前駆体である[6]。
ニッチな使用
[編集]LiHMDSは揮発性であり、リチウム化合物の原子層堆積での使用が検討されている[7]。
構造
[編集]多くの有機リチウム化合物と同様に、リチウム ビス (トリメチルシリル) アミドは溶液中で凝集体を形成する可能性がある。凝集の程度は溶媒に依存する。エーテル[8]やアミン[9] などの配位溶媒中では、モノマーかダイマーの形をとるのが一般的である。モノマーおよびダイマー状態では、1つまたは2つの溶媒分子がリチウム中心に結合する。ドナーベースとしてアンモニアを使用すると、リチウム ビス (トリメチルシリル) アミドは、分子間水素結合によって安定化される三溶媒和モノマーを形成する[10][11]。芳香族やペンタンなどの非配位性溶媒では、三量体を含む複合体オリゴマーが優勢である[9]。固体状の場合の構造は三量体である[12]。

| ||||
 LiHMDS adduct with TMEDA |
 THF solvated dimer: (LiHMDS)2•THF2 |
 Trimer, solvent free: (LiHMDS)3 | ||
関連項目
[編集]脚注
[編集]- ^ Amonoo-Neizer, E. H.; Shaw, R. A.; Skovlin, D. O.; Smith, B. C. (1966). “Lithium Bis(Trimethylsilyl)Amide and Tris(Trimethylsilyl)Amine”. Inorg. Synth.. Inorganic Syntheses 8: 19–22. doi:10.1002/9780470132395.ch6. ISBN 978-0-470-13239-5.
- ^ a b Danheiser, R. L.; Miller, R. F.; Brisbois, R. G. (1990). "Detrifluoroacetylative Diazo Group Transfer: (E)-1-Diazo-4-phenyl-3-buten-2-one". Organic Syntheses (英語). 73: 134.; Collective Volume, vol. 9, p. 197
- ^ a b Wu, George; Huang, Mingsheng (July 2006). “Organolithium Reagents in Pharmaceutical Asymmetric Processes”. Chemical Reviews 106 (7): 2596–2616. doi:10.1021/cr040694k. PMID 16836294.
- ^ Fraser, Robert R.; Mansour, Tarek S.; Savard, Sylvain (August 1985). “Acidity measurements on pyridines in tetrahydrofuran using lithiated silylamines”. The Journal of Organic Chemistry 50 (17): 3232–3234. doi:10.1021/jo00217a050.
- ^ Maaninen, A.; Shvari, J.; Laitinen, R. S.; Chivers, T (2002). Coucouvanis, Dimitri. ed. “Compounds of General Interest”. Inorganic Syntheses (New York: John Wiley & Sons, Inc.) 33: 196–199. doi:10.1002/0471224502.ch4.
- ^ Michael Lappert, Andrey Protchenko, Philip Power, Alexandra Seeber (2009). Metal Amide Chemistry. Weinheim: Wiley-VCH. doi:10.1002/9780470740385. ISBN 0-470-72184-7
- ^ Hämäläinen, Jani; Holopainen, Jani; Munnik, Frans; Hatanpää, Timo; Heikkilä, Mikko; Ritala, Mikko; Leskelä, Markku (2012). “Lithium Phosphate Thin Films Grown by Atomic Layer Deposition”. Journal of the Electrochemical Society 159 (3): A259–A263. doi:10.1149/2.052203jes.
- ^ Lucht, Brett L.; Collum, David B. (1995). “Ethereal Solvation of Lithium Hexamethyldisilazide: Unexpected Relationships of Solvation Number, Solvation Energy, and Aggregation State”. Journal of the American Chemical Society 117 (39): 9863–9874. doi:10.1021/ja00144a012.
- ^ a b Lucht, Brett L.; Collum, David B. (1996). “Lithium Ion Solvation: Amine and Unsaturated Hydrocarbon Solvates of Lithium Hexamethyldisilazide (LiHMDS)”. Journal of the American Chemical Society 118 (9): 2217–2225. doi:10.1021/ja953029p.
- ^ Neufeld, R.; Michel, R.; Herbst-Irmer, R.; Schöne, R.; Stalke, D. (2016). “Introducing a Hydrogen-Bond Donor into a Weakly Nucleophilic Brønsted Base: Alkali Metal Hexamethyldisilazides (MHMDS, M = Li, Na, K, Rb and Cs) with Ammonia”. Chem. Eur. J. 22: 12340–12346. doi:10.1002/chem.201600833. PMID 27457218.
- ^ Neufeld, R.: DOSY External Calibration Curve Molecular Weight Determination as a Valuable Methodology in Characterizing Reactive Intermediates in Solution. In: eDiss, Georg-August-Universität Göttingen. 2016.
- ^ Rogers, Robin D.; Atwood, Jerry L.; Grüning, Rainer (1978). “The crystal structure of N-lithiohexamethyldisilazane, [LiN(SiMe3)2]3”. J. Organomet. Chem. 157 (2): 229–237. doi:10.1016/S0022-328X(00)92291-5.