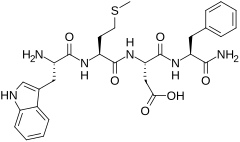
CCK-4
 | |
| IUPAC命名法による物質名 | |
|---|---|
| |
| 薬物動態データ | |
| 生物学的利用能 | 100% |
| 代謝 | plasma protease enzymes |
| 半減期 | 13 minutes |
| 排泄 | N/A |
| 識別 | |
| CAS番号 |
1947-37-1 |
| ChemSpider |
393888 |
| UNII |
0OL293AV80 |
| ChEBI | CHEBI:137728 |
| 化学的データ | |
| 化学式 | C29H35N5O7S |
| 分子量 | 597.681 g/mol |
| |
CCK-4またはコレシストキニンテトラペプチド(Cholecystokinin tetrapeptide)は、ホルモンの1つコレシストキニンの断片であるペプチドである。アミノ酸配列は、Trp-Met-Asp-Phe-NH2。消化器系及び中枢神経系に様々な作用を示すコレシストキニンとは異なり、CCK-4は主に不安惹起薬として脳に作用する。消化器系に対する作用は多少残存しているものの、CCK-8や全長のポリペプチドCCK-58ほど強くはない。
CCK-4は、わずか50 μgの投与でヒトに確実に深刻な不安症状を引き起こし[1]、抗不安薬の試験のためにパニック発作を引き起こすのに用いられる[2][3][4][5]。ペプチドであるため、注射によって投与する必要があり、体内に入るとすぐに分解されるため、作用の持続時間は長くはないが[6]、性質を改善した多くの合成アナログが知られている[7][8][9][10][11][12][13][14][15][16][17]。
出典
[編集]- ^ Daniela Eser (2005). “Panic Induction with Cholecystokinin-Tetrapeptide (CCK-4) Increases Plasma Concentrations of the Neuroactive Steroid 3α, 5α Tetrahydrodeoxycorticosterone (3α, 5α-THDOC) in Healthy Volunteers”. Neuropsychopharmacology 30 (1): 192-195. doi:10.1038/sj.npp.1300572. PMID 15467707.
- ^ Bradwejn J. (July 1993). “Neurobiological investigations into the role of cholecystokinin in panic disorder”. Journal of Psychiatry and Neuroscience 18 (4): 178-188. PMC 1188527. PMID 8104032.
- ^ “Functional magnetic resonance imaging characterization of CCK-4-induced panic attack and subsequent anticipatory anxiety”. NeuroImage 31 (3): 1197-1208. (July 2006). doi:10.1016/j.neuroimage.2006.01.035. PMID 16600640.
- ^ “Evaluation of the CCK-4 model as a challenge paradigm in a population of healthy volunteers within a proof-of-concept study”. Psychopharmacology 192 (4): 479-487. (July 2007). doi:10.1007/s00213-007-0738-7. PMID 17318504.
- ^ “Functional neuroanatomy of CCK-4-induced panic attacks in healthy volunteers”. Human Brain Mapping 30 (2): 511-22. (December 2007). doi:10.1002/hbm.20522. PMID 18095276.
- ^ “Degradation of cholecystokinin octapeptide, related fragments and analogs by human and rat plasma in vitro”. Regulatory Peptides 4 (3): 127-139. (August 1982). doi:10.1016/0167-0115(82)90080-5. PMID 6291099.
- ^ “Structure-based design of new constrained cyclic agonists of the cholecystokinin CCK-B receptor”. Journal of Medicinal Chemistry 40 (5): 647-58. (February 1997). doi:10.1021/jm9603072. PMID 9057851.
- ^ “Replacement of glycine with dicarbonyl and related moieties in analogues of the C-terminal pentapeptide of cholecystokinin: CCK(2) agonists displaying a novel binding mode”. Journal of Medicinal Chemistry 43 (20): 3614-23. (October 2000). doi:10.1021/jm0000416. PMID 11020275.
- ^ “Involvement of D2 dopamine receptors in the opposing effects of two CCK-B agonists in a spatial recognition memory task: role of the anterior nucleus accumbens”. Psychopharmacology 153 (2): 170-9. (January 2001). doi:10.1007/s002130000517. PMID 11205416.
- ^ “How a single inversion of configuration leads to a reversal of the binding mode: proposal of a novel arrangement of CCK2 ligands in their receptor, and contribution to the development of peptidomimetic or non-peptide CCK2 ligands”. European Journal of Medicinal Chemistry 38 (7-8): 671-86. (2003). doi:10.1016/S0223-5234(03)00112-0. PMID 12932898.
- ^ “New CCK2 agonists confirming the heterogeneity of CCK2 receptors: characterisation of BBL454”. Naunyn-Schmiedeberg's Archives of Pharmacology 370 (5): 404-13. (November 2004). doi:10.1007/s00210-004-0969-7. PMID 15480577.
- ^ “[Biological activity of cholecystokinin-(30-33) tetrapeptide analogs]” (Russian). Bioorganicheskaia Khimiia 31 (2): 130-9. (2005). PMID 15889786.
- ^ “[Effect of a cholecystokinin tetrapeptide analogue on opioid reception under acute and chronic morphine administration]” (Russian). Bioorganicheskaia Khimiia 32 (3): 276-83. (2006). PMID 16808170.
- ^ “Structure-activity relationships of bifunctional peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors”. Journal of Medicinal Chemistry 49 (10): 2868-75. (May 2006). doi:10.1021/jm050921q. PMC 1484468. PMID 16686530.
- ^ Noble F (2007). “Pharmacology of CCKRs and SAR studies of peptidic analog ligands”. Current Topics in Medicinal Chemistry 7 (12): 1173-9. doi:10.2174/156802607780960447. PMID 17584139.
- ^ “Strategies for design of non peptide CCK1R agonist/antagonist ligands”. Current Topics in Medicinal Chemistry 7 (12): 1180-94. (2007). doi:10.2174/156802607780960537. PMID 17584140.
- ^ “Strategies for the design of non-peptide CCK2 receptor agonist and antagonist ligand”. Current Topics in Medicinal Chemistry 7 (12): 1195-204. (2007). doi:10.2174/156802607780960500. PMID 17584141.