アカンプロサート
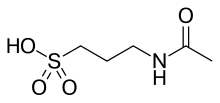 | |
| IUPAC命名法による物質名 | |
|---|---|
| |
| 臨床データ | |
| 胎児危険度分類 | |
| 法的規制 | |
| 薬物動態データ | |
| 生物学的利用能 | 11%[1] |
| 血漿タンパク結合 | 無視できる[1] |
| 代謝 | 代謝されない[1] |
| 半減期 | 20 から 33 時間[1] |
| 排泄 | 腎[1] |
| データベースID | |
| CAS番号 |
77337-76-9 |
| ATCコード | N07BB03 (WHO) |
| PubChem | CID: 155434 |
| DrugBank |
DB00659 |
| ChemSpider |
136929 |
| UNII |
N4K14YGM3J |
| KEGG |
D07058 |
| ChEBI |
CHEBI:51042 |
| ChEMBL |
CHEMBL1201293 |
| 化学的データ | |
| 化学式 | C5H11NO4S |
| 分子量 | 181.211 g/mol |
| |
アカンプロサート(Acamprosate、N-アセチルホモタウリン)[2]は日本国内では2013年から日本新薬株式会社よりレグテクトの商標名で発売され、海外ではCampral、の商標名で販売されているアルコール依存症の治療薬である[3]。中枢神経系に作用して、飲酒欲求を抑える作用を持つ。
アカンプロサートは飲酒欲求を抑える薬であり、急性期の解毒薬ではない。アルコール依存症で乱された脳の化学的なバランスを安定化させる薬物であると考えられている。作用機序はグルタミン酸受容体の一種であるNMDA受容体を阻害し、GABA受容体の一種であるGABAA受容体を刺激することによる[4]。アカンプロサートは断酒会などの自助グループに参加し断酒を実行できて初めて効果を示すと報告されている[5][6]。WHOガイドラインではアルコール依存症の再発予防薬とされている[3]。
重篤な副作用として下痢、アレルギー反応、不整脈、血圧の変化などが判明している。他の副作用としては頭痛、不眠、インポテンスが知られている[7]。アカンプロサートは、本剤の成分に対し過敏症の既往歴のある患者、および高度の腎障害のある患者(排泄遅延により、高い血中濃度が持続するおそれがある)には投与してはならない[8]。
アカンプロサートは日本ではアルコール依存症・断酒補助剤"レグテクト錠"の商標名で、日本新薬株式会社によって生産販売されている。アメリカ合衆国ではCampralの商標名でForest Laboratoriesによって生産販売されている。アメリカ以外ではMerck KGaAが販売している。アカンプロサートカルシウム塩として333mgを含有する白色無臭の錠剤として販売されており、一錠にはアカンプロサートとして300mg相当が含まれる[1]。
薬理学的な作用
[編集]
GABAA受容体に対する作用
[編集]エタノールは、GABAA受容体に結合して抑制性の神経伝達物質であるGABAの作用を増強することによって中枢神経系に作用する(つまり、エタノールはGABAA受容体に対して正のアロステリックモジュレーターとして働く)。 慢性的なアルコール依存状態では、GABAA受容体がダウンレギュレーションを受ける(つまり、GABA伝達系の抑制作用に対して感受性が低下した状態になる)ことが耐性獲得の機序の一つになっていると考えられている。アルコールを飲んでいない状態では、これらのダウンレギュレートされたGABAA受容体は通常の神経伝達で放出される濃度のGABAには反応しなくなり、通常起こるはずのGABAに対する反応が起きなくなる。この状態では通常のGABAの作用である、活動電位の発生を抑制する作用が発揮できなくなるため、交感神経の興奮がGABA性の神経伝達によって抑制されなくなってしまう。アカンプロサートの作用として考えられていることの少なくとも一部はGABA受容体に対してアゴニストとして働くことによるものと考えられている。
NMDA型グルタミン酸受容体に対する作用
[編集]エタノールの中枢神経系に対する他の作用として、NMDA受容体の抑制作用が挙げられる。慢性的にアルコールを乱用するとNMDA受容体が過剰に作られ(アップレギュレーション)てしまう。このため、突然断酒すると正常脳より過多となっているNMDA受容体の活性化が起こることで、振戦譫妄(しんせんせんもう)や興奮性神経細胞死などを起こす原因となっていると考えられている[9]。アルコールの離脱がグルタミン酸のような興奮性神経伝達物質の放出を急激に高め、結果としてNMDA受容体を活性化することになる[10]。アカンプロサートはこのグルタミン酸の急激な上昇を抑制する[11]。in vitroの実験系の結果ではあるが、アカンプロサートはエタノール離脱によって引き起こされる神経細胞死からも神経細胞を保護する効果を示す[12]。エタノール離脱と組み合わせたグルタミン酸の神経毒性に対しても保護効果を示した[13]。
Cedars-Sinai Medical Centerにある、脳研究所から発表されたある研究によれば、アカンプロサートにはヒトでニコチン依存の治療を行う際にも有用性がありそうだと思われる[14]。
神経保護作用の可能性
[編集]患者達が断酒を継続するのを助ける明らかな効果に加えて、アカンプロサートには神経保護作用があるらしいと報告されている(つまり、アカンプロサートはアルコール離脱またはその他の侵害刺激によって引き起こされる神経細胞障害と神経細胞死とから神経細胞を保護する作用を持つ)[2][11]例えば、培養神経細胞を用いた研究からアカンプロサートは虚血(血流が不足する病的な状態)によって引き起こされる障害から神経細胞を保護する作用を持つことが報告されている[15]。また別の研究では、アカンプロサートは、イボテン酸(グルタミン酸受容体を有害なレベルまで過剰興奮させる興奮毒性を持つ毒物)の注入によって実験的に引き起こされる脳障害からハムスターの乳児を保護する作用を示した[16]。
ブラジルで行われたある研究によれば、アカンプロサートは耳鳴(聴力喪失後の長期間持続する耳鳴)の治療に効果を持つ可能性がある[17]。
承認
[編集]米国のFDAは2004年7月に本剤を認可したが、ヨーロッパでは1989年以来合法的に用いられてきた。認可の後、FDAは次のような声明を発表した。
Campralの作用機序は完全には理解されていないが、Campralはアルコール乱用に関係する脳の経路に作用すると考えられている。Campral は、既に断酒に成功した者を含むアルコール依存症の患者も対象とした複数のプラセボ対照臨床試験の結果安全かつ効果的であると証明されている。Campralはプラセボと比較して断酒期間を維持する効果が優れており、治療期間全体に渡り断酒できた患者の割合がアカンプロサート-治療群で高かったことでもその効果が示されていた。 Campralは依存性を起こす物質ではなく、臨床試験中薬効を維持できた。最も高頻度で見られる副作用は、頭痛、下痢、腹部膨満および悪心であったと報告されている。[18]
英国では、英国国立医療技術評価機構のガイドラインで推奨されている[19]。
臨床試験結果
[編集]Scripps Research Instituteはアカンプロサート投与群とプラセボ対照群を心理療法群と組み合わせた二重盲検法によるアルコール依存の治療臨床試験を実施した。アカンプロサート投与群で断酒成功日数の延長が見られたため、アカンプロサートは安全かつ有効であると結論づけている[20]。
BrisbaneのPrincess Alexandra Hospitalで行われた臨床試験では、12週間の試験期間中アカンプロサート投与群, ナルトレキソン投与群, 両剤使用群の比較および認知行動療法を受けた群との比較が行われた[21]。この研究では、下表に示すように、どちらの薬物も単剤使用より両剤使用の方が好まれ、かつ優れた治療結果をもたらした。
| 参加者の割合 | 断酒率 | 断酒成功日数の平均1 | 断酒失敗までの経過日数の平均1
| |
|---|---|---|---|---|
| アカンプロサート投与群 | 66.1% | 50.8% | 45.07 日 | 26.79 日 |
| ナルトレキソン投与群 | 79.7% | 66.1% | 49.95 日 | 26.7 日 |
| 両剤投与群 | 83.1% | 67.8% | 53.58 日 | 37.32 日 |
このデータには、84日間の全期間中にわたって断酒できなかった患者のデータも含まれている。
歴史
[編集]- 1984年:フランスのメラム社がてんかんおよびアルコール依存症の治療薬として開発
- 1987年:同社、フランスにて同薬剤の仮認可を取得
- 1989年:同社、同薬剤をフランス薬剤市場にその後ヨーロッパ規模での認可に向けてフランスのリファ社(メルク社の孫会社)がアカンプロサートの所有権を取得
- 1995年:ドイツにて「キャンプラル」として認可
- 1996年:リファ社、アカンプロサート含有薬剤「キャンプラル」をドイツ市場に
- 2004年:7月、アメリカにてアルコール依存症患者の断酒継続のために、「キャンプラル」が認可され、治療が始まった。
- 2013年:3月、日本で「レグテクト」が認可され、治療が始まった。
脚注
[編集]- ^ a b c d e f g “Campral Description” (PDF). 2006年3月18日時点のオリジナルよりアーカイブ。2006年4月2日閲覧。
- ^ a b Mann K, Kiefer F, Spanagel R, Littleton J (July 2008). “Acamprosate: recent findings and future research directions”. Alcohol. Clin. Exp. Res. 32 (7): 1105–10. doi:10.1111/j.1530-0277.2008.00690.x. PMID 18540918.
- ^ a b mhGAP Intervention Guide for mental, neurological and substance use disorders in non-specialized health settings (Report). 世界保健機関. 2010. ALC1. ISBN 9789241548069。
- ^ Williams, SH. (2005). “Medications for treating alcohol dependence”. American Family Physician 72 (9): 1775–1780. PMID 16300039.
- ^ Mason, BJ (2001). “Treatment of alcohol-dependent outpatients with acamprosate: a clinical review.”. The Journal of clinical psychiatry 62 Suppl 20: 42–8. PMID 11584875.
- ^ GABA Agonist (Acamprosate) for Alcohol Treatment, Alcohol Rehab Thailand
- ^ “Acamprosate”. drugs.com (2005年3月25日). 2006年12月22日時点のオリジナルよりアーカイブ。2007年1月8日閲覧。
- ^ “Acamprosate Oral - Who should not take this medication?”. WebMD.com. 2007年1月8日閲覧。
- ^ Tsai, G; Coyle, JT (1998). “The role of glutamatergic neurotransmission in the pathophysiology of alcoholism”. Annual review of medicine 49: 173–84. doi:10.1146/annurev.med.49.1.173. PMID 9509257.
- ^ Tsai, GE; Ragan, P; Chang, R; Chen, S; Linnoila, VM; Coyle, JT (1998). “Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal”. The American Journal of Psychiatry 155 (6): 726–32. PMID 9619143.
- ^ a b De Witte, P; Littleton, J; Parot, P; Koob, G (2005). “Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action”. CNS Drugs 19 (6): 517–37. PMID 15963001.
- ^ Mayer, S; Harris, BR; Gibson, DA; Blanchard, JA; Prendergast, MA; Holley, RC; Littleton, J (2002). “Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus”. Alcoholism, clinical and experimental research 26 (10): 1468–78. doi:10.1097/01.ALC.0000033261.14548.D2. PMID 12394279.
- ^ Al Qatari, M; Khan, S; Harris, B; Littleton, J (2001). “Acamprosate is neuroprotective against glutamate-induced excitotoxicity when enhanced by ethanol withdrawal in neocortical cultures of fetal rat brain”. Alcoholism, clinical and experimental research 25 (9): 1276–83. doi:10.1111/j.1530-0277.2001.tb02348.x. PMID 11584146.
- ^ Pechnick, RN; Manalo, CM; Lacayo, LM; Vit, JP; Bholat, Y; Spivak, I; Reyes, KC; Farrokhi, C (2011). “Acamprosate attenuates cue-induced reinstatement of nicotine-seeking behavior in rats”. Behavioural Pharmacology 22 (3): 222–7. doi:10.1097/FBP.0b013e328345f72c. PMID 21522053.
- ^ Engelhard, K; Werner C, Lu H, Mollenberg O, Zieglgansberger W, Kochs E (2006). “The neuroprotective effect of the glutamate antagonist acamprosate following experimental cerebral ischemia. A study with the lipid peroxidase inhibitor u-101033e”. Anaesthesist 49 (9): 816–821. PMID 11076270.
- ^ Adde-Michel, C; Hennebert O, Laudenbach V, Marret S, Leroux P (2005). “Effect of acamprosate on neonatal excitotoxic cortical lesions in in utero alcohol-exposed hamsters”. Neuroscience Letters 374 (2): 109–112. doi:10.1016/j.neulet.2004.10.037. PMID 15644274.
- ^ Azevedo AA, Figueiredo RR (2005). “Tinnitus treatment with acamprosate: double-blind study”. Braz J Otorhinolaryngol 71 (5): 618–23. doi:10.1590/S0034-72992005000500012. PMID 16612523.
- ^ “FDA Approves New Drug for Treatment of Alcoholism”. FDA Talk Paper. Food and Drug Administration (2004年7月29日). 2008年1月17日時点のオリジナルよりアーカイブ。2009年8月15日閲覧。
- ^ CG115 - Alcohol-use disorders: diagnosis, assessment and management of harmful drinking and alcohol dependence (Report). 英国国立医療技術評価機構. February 2011. Chapt.1.3.6.
- ^ Mason, BJ; Goodman AM, Chabac S, Lehert P (2006). “Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: The role of patient motivation”. J Psychiatr Res 40 (5): 383–393. doi:10.1016/j.jpsychires.2006.02.002. PMID 16546214.
- ^ Feeney, GF; Connor JP, Young RM, Tucker J, McPherson A (2006). “Combined acamprosate and naltrexone, with cognitive behavioural therapy is superior to either medication alone for alcohol abstinence: A single centre's experience with pharmacotherapy”. Alcohol Alcohol 41 (3): 321–327. doi:10.1093/alcalc/agl007. PMID 16467406.