スルホレン
| スルホレン[1] | |
|---|---|
|
|
2,5-Dihydrothiophene 1,1-dioxide | |
2,5-Dihydro-1H-1λ6-thiophene-1,1-dione | |
別称 Butadiene sulfone 3-Sulfolene | |
| 識別情報 | |
| CAS登録番号 | 77-79-2 |
| PubChem | 6498 |
| ChemSpider | 6253 |
| UNII | Z6003L44MN |
| |
| 特性 | |
| 化学式 | C4H6O2S |
| モル質量 | 118.15 g mol−1 |
| 融点 |
65 - 66 °C, 272 K, -22 °F |
| 特記なき場合、データは常温 (25 °C)・常圧 (100 kPa) におけるものである。 | |
スルホレン(Sulfolene)は、スルホン基を持つ環状有機化合物である。白色無臭結晶性で無期限に保存可能な固体であり、水や多くの有機溶媒に可溶である[2]。ブタジエン合成の材料として用いられる[3]。
生成
[編集]
スルホレンは1,3-ブタジエンと二酸化硫黄の可逆キレトロピー反応により合成される。これらの化合物は、まずオートクレーブ中で約-20℃で、少量のフェノール重合阻害剤(ヒドロキノンやピロガロール)の存在下、過剰量の二酸化硫黄と混合され、その後、室温に数日置くか、130℃で30分間加熱される[4]。
反応
[編集]酸塩基反応
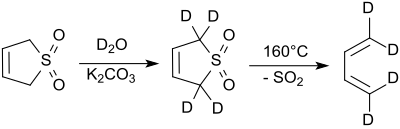
[編集]2位及び5位のプロトンは、塩基性条件下で急速に重水と置き換わる[7]。シアン化ナトリウムはこの反応を触媒する[8]。
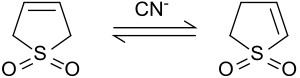
2-スルホレンへの異性化
[編集]塩基性条件下またはシアン化イオンにより触媒された場合、3-スルホレンは、2-スルホレンと3-スルホレンの混合物に異性化する。2-スルホレンと3-スルホレンの比は、シアニドとスルホレンの存在比に依る[8]。
50℃では、42%の3-スルホレンと58%の2-スルホレンを含む平衡混合物が得られる。[9]3-スルホレンは80℃以上で熱分解するため、熱力学的により安定な2-スルホレンは、100℃で数日間加熱することで、異性体混合物から白い板状の純粋な物質(融点48-49℃)として単離することができる[10]。
水素化
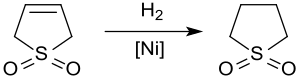
[編集]触媒水素化により、石油化学工業において炭化水素流からの芳香族化合物の抽出に用いられる溶媒であるスルホランが得られる。約20気圧60℃でラネー合金により3-スルホレンを水素化すると、65%の収率でスルホランが得られる[11]。
ハロゲン化
[編集]3-スルホレンは水溶液中で臭素と反応し、3,4-ジブロモテトロヒドロチオフェン-1,1-ジオキシドを与え、これは、炭酸銀(I)で脱臭化水素してチオフェン-1,1-ジオキシドとなる[5]。非常に反応性の高いチオフェン-1,1-ジオキシドは、3,4-bis(ジメチルアミノ)テトラヒドロチオフェン-1,1-ジオキシドの形成後、ヨードメタンによる二重四級化と水酸化銀によるホフマン脱離によっても生成する[10]。
より簡便な二段階合成は、テトラヒドロフラン中での粉末水酸化ナトリウムか[12]、または超音波分散した金属カリウムによる[13]、3,4-ジブロモテトロヒドロチオフェン-1,1-ジオキシドの二重脱臭化水素による。
ディールス・アルダー反応
[編集]3-スルホレンは主にブタジエンの代用として価値を持つ[2][3]。1,3-ブタジエンのin situでの製造と即時の消費は、室温で気体であるジエンとの接触を大幅に回避する。費用以外の欠点の1つは、発生した二酸化硫黄が酸と反応しやすい物質と副反応を引き起こす可能性があることである。
1,3-ブタジエンと反応性の低いジエノファイルとのディールス・アルダー反応は、100℃以上での長時間の過熱を必要とし、危険である。純粋なブタジエンを用いた場合、高圧に耐えられる特殊な装置が必要である。
スルホレンでは、遊離したジエンが付加環化反応で消費されるため、ブタジエンの圧力の上昇は予想できない。したがって、可逆的押出反応の平衡は、内部の「安全弁」として機能する[14]。
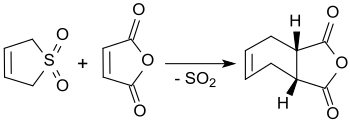
3-スルホレンは、沸騰キシレン中で無水マレイン酸と反応し、cis-4-シクロヘキセン-1,2-ジカルボン酸無水物となり、その収率は90%にもなる[3]。
3-スルホレンは、110℃、trans型でフマル酸ジエチル等のジエノファイルとも反応し、二酸化硫黄を脱離して66-73%の収率でtrans-4-シクロヘキセン-1,2-ジカルボン酸ジエチルエステルが得られる[15]。
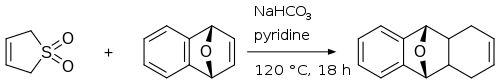
沸騰キシレン中で6,7-ジブロモ-1,4-ジヒドロナフタレンと3-スルホレンが反応し、三環式付加物が得られる。この前駆体は、過塩素酸で処理した後、最後のステップで2,3-ジクロロ-5,6-ジシアノ-p-ベンゾキノンで脱水素化されて2,3-ジブロモアントラセンになるジブロモジヒドロアントラセンを生成する[16]。
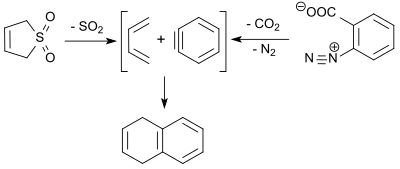
3-スルホレンの逆キレトロピー反応で得られる1,3-ブタジエンは、ベンザインとディールス・アルダー反応し、9%の収率で、1,4-ジヒドロナフタレンを得る[17]。
ジエノフィルとしての2-及び3-スルホレン
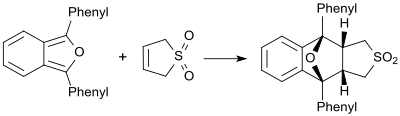
[編集]例えば、1,3-ジフェニルイソベンゾフラン等の非常に反応性の高いジエンが存在する時には、ブタジエンスルホンはジエノフィルとして振る舞い、対応するディールス・アルダー付加物を形成する[18]。
早くも1938年には、クルト・アルダーらは、異性体の2-スルホレンと1,3-ブタジエンの、また2-スルホレンとシクロペンタジエンのディールス・アルダー付加物を報告した[19]。
その他の環化付加反応
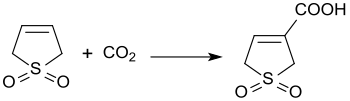
[編集]3気圧下での3-スルホレンと二酸化炭素との塩基触媒反応により、3-スルホレン-3-カルボン酸が45%の収率で生成する[20]。
また、ジアゾメタンとともに、3-スルホレンは1,3-双極子付加環化反応を起こす[21]。
重合
[編集]1935年、ヘルマン・シュタウディンガーらは、室温でのブタジエンと二酸化硫黄の反応により二次生成物として、3-スルホレンへの付加物が生成することを発見した。この二次生成物は、アモルファス固体ポリマーである。過酸化物含有ジエチルエーテル中で3-スルホレンのフリーラジカル重合により、最大50%の不溶性高分子量ポリスルホレンが得られる。このポリマーは、硫酸や硝酸による分解に対する抵抗性を持つ[6]。
その後の研究によって、3-スルホレンの重合は、ラジカル開始剤アゾビスイソブチロニトリルの存在下、100℃以上の音頭で開始することが明らかとなった[22]。3-スルホレンは、ビニル化合物と共重合しないが、逆に2-スルホレンは単重合はせず、アクリロニトリルやビニル酢酸等のビニル化合物と共重合する。
再利用可能触媒としての3-スルホレン
[編集]3-スルホレンと1,3-ブタジエン及び二酸化硫黄の間の相互変換の可逆性は、スルホレンを、よく使われるが分離が難しく再利用できないジメチルスルホキシドの代用として、再利用可能な双極性非プロトン溶媒として用いることの可能性を示している[23]。モデル反応として、ベンジルアジドと4-トルエンスルホン酸シアニドが1-ベンジル-5-(4-トルエンスルホニル)テトラゾールを形成する反応が調べられている。テトラゾールの形成は、ベンジルアジドを単離しないワンポット反応で、72%の収率で合成することもできる。
反応後、135℃に加熱して3-スルホレン溶媒を分解し、揮発性のブタジエンと二酸化硫黄を-76℃のコールドトラップで沈殿させる。重合阻害剤としてヒドロキノンを添加した後、室温まで加熱することで、等量の3-スルホレンが再生する。しかし、液体状態の温度範囲が64℃から約100℃と狭いため、扱いやすく低価格で環境適合性の高いジメチルスルホキシドの代用として産業において実用利用することについては、疑問が持たれている。
利用
[編集]上述の有機合成における汎用性の他に、スルホレンは、電解フッ素化の添加物として用いられ、パーフルオロオクタンスルホニルの生産量を70%増やすことができる[24]。無水フッ化水素に溶解し、電解質溶液の導電率を増加させる[24]。この際には、開環の後、フッ素化され、パーフルオロブタンスルホニルフルオリドを形成する。
脚注
[編集]- ^ Sulfolene at Sigma-Aldrich
- ^ a b J. M. McIntosh (2001). “3-Sulfolene”. E-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rs130.
- ^ a b c Sample, Thomas E.; Hatch, Lewis F (Jan 1968). “3-Sulfolene: A Butadiene Source for a Diels-Alder Synthesis”. Journal of Chemical Education 45 (1): 55. doi:10.1021/ed045p55.
- ^ Houben-Weyl (1955). Volume IX: Sulfur, Selenium, Tellurium Compounds. Methods of Organic Chemistry (4th ed.). Stuttgart: Georg Thieme Verlag. p. 237. ISBN 978-3-13-208104-8
- ^ a b DE 506839, H. Staudinger, "Verfahren zur Darstellung von monomolekularen Reaktionsprodukten von ungesattigten Kohlenwasserstoffen der Butadienreihe mit Schwefeldioxyd"
- ^ a b H. Staudinger; B. Ritzenthaler (1935). “Uber hochmolekulare Verbindungen, 104. Mitteil.: Uber die Anlagerung von Schwefeldioxyd an Athylen-Derivate” (ドイツ語). Chemische Berichte 68 (3): 455-471. doi:10.1002/cber.19350680317.
- ^ J. Leonard; A. B. Hague; J. A. Knight (1998). “6. Preparation of substituted 3-sulfolenes and their use as precursors for Diels-Alder dienes”. Organosulfur Chemistry. 2 (4th ed.). San Diego: Academic Press, Inc.. p. 241. ISBN 0-12-543562-2
- ^ a b US 4187231, R. L. Cobb, "Cyanide-catalyzed isomerization and deuterium exchange with alpha- and beta-sulfolenes"
- ^ C. D. Broaddus (1968). “Equilibria and base-catalyzed exchange of substituted olefins”. Accounts of Chemical Research 1 (8): 231-238. doi:10.1021/ar50008a002.
- ^ a b W. J. Bailey; E. W. Cummins (1954). “Cyclic dienes. III. The synthesis of thiophene-1,1-dioxide”. Journal of the American Chemical Society 76 (7): 1932-1936. doi:10.1021/ja01636a058.
- ^ US 4286099, M. E. Nash, E. E. Huxley, "Sulfolene hydrogenation"
- ^ D. M. Lemal; G. D. Goldman (1988). “Synthesis of azulene, a blue hydrocarbon”. Journal of Chemical Education 65 (10): 923. doi:10.1021/ed065p923.
- ^ T.-S. Chou; M.-M. Chen (1987). “Chemoselective reactions of ultrasonically dispersed potassium with some brominated hydrothiophene-S,S-dioxides”. Heterocycles 26: 2829-2834. doi:10.3987/R-1987-11-2829.
- ^ M. A. Filatov; S. Baluschev; I. Z. Ilieva; V. Enkelmann; T. Miteva; K. Landfester; S. E. Aleshchenkov; A. V. Cheprakov (2012). “Tetraaryltetraanthra[2,3]porphyrins: Synthesis, Structure, and Optical Properties”. The Journal of Organic Chemistry 77 (24): 11119-11131. doi:10.1021/jo302135q. PMID 23205621.
- ^ "Diethyl trans-Δ4-tetrahydrophthalate". Organic Syntheses (英語). 50. doi:10.15227/orgsyn.050.0043。
- ^ C.-T. Lin, T.-C. Chou (1988). “Synthesis of 2,3-dibromoanthracene”. Synthesis 1988: 628-630. doi:10.1055/s-1988-27659.
- ^ L. F. Hatch, D. Peter (1968). “Reaction of benzyne with butadiene”. Chemical Communications 23 (23): 1499. doi:10.1039/C19680001499.
- ^ M. P. Cava, J. P. VanMeter (1969). “Condensed cyclobutane aromatic compounds. XXX. Synthesis of some unusual 2,3-naphthoquinonoid heterocycles. A synthetic route to derivatives of naphtho[2,3-b]biphenylene and anthra[b]cyclobutene”. The Journal of Organic Chemistry 34: 538-545. doi:10.1021/jo01255a012.
- ^ K. Alder; H. F. Rickert; E. Windemuth (1938). “Zur Kenntnis der Dien-Synthese, X. Mitteil.: Uber die Dien-Synthese mit α, β-ungesattigten Nitrokorpern, Sulfonen und Thio-Athern”. Chemische Berichte 71: 2451-2461. doi:10.1002/cber.19380711206.
- ^ G. S. Andrade (2003). “The one-pot synthesis and Diels-Alder Reactivity of 2,5-dihydrothiophene-1,1-dioxide-3-carboxylic acid”. Synthetic Communications 33: 3643-3650. doi:10.1081/SCC-120024845.
- ^ M. E. Brant; J. E. Wulff (2016). “3-Sulfolenes and their derivatives: Synthesis and applications”. Synthesis 48 (1): 1-17. doi:10.1055/s-0035-1560351.
- ^ E. J. Goethals (1967). “On the polymerization and copolymerization of sulfolenes”. Macromolecular Chemistry and Physics 109 (1): 132-142. doi:10.1002/macp.1967.021090113.
- ^ Y. Huang (2015). “Butadiene sulfone as 'volatile', recyclable dipolar, aprotic solvent for conducting substitution and cycloaddition reactions”. Sustainable Chemical Processes 3 (13). doi:10.1186/s40508-015-0040-7.
- ^ a b Lehmler HJ (March 2005). “Synthesis of environmentally relevant fluorinated surfactants-a review”. Chemosphere 58 (11): 1471-96. doi:10.1016/j.chemosphere.2004.11.078. PMID 15694468.
関連文献
[編集]- DE 506839, H. Staudinger, "Verfahren zur Darstellung von monomolekularen Reaktionsprodukten von ungesattigten Kohlenwasserstoffen der Butadienreihe mit Schwefeldioxyd"