セレノール

セレノール(selenol)類は、C-Se-Hという原子の連結を持つ官能基を含む有機化合物である。セレノール類はセレノメルカプタン類やセレノチオール類と呼ばれることもある。セレノール類は有機セレン化合物の主要な分類の一つである。最も良く知られたセレノール類はアミノ酸の一種であるセレノシステインである。
構造、結合、性質
[編集]セレノール類は構造的にチオール類と類似しているが、C-Se結合は196 pmと約8%長い。C-Se-Hの角度はセレン化水素(H2Se)のように90° に近付いている。この結合はSe上のほぼ純粋なp軌道を含んでおり、ゆえに角度が90° に近い。Se-H結合エネルギーはS-H結合よりも弱く、その結果としてセレノール類は容易に酸化され、H-原子の供与体となる。またSeの結合の相対的弱さを反映して、セレノール類はチオール類よりも約1000倍強い酸である。CH3SeHとCH3SHのpKaの値はそれぞれ5.2と8.3である。脱プロトン化によってセレノラート陰イオン(RSe−)が生じる。セレノラートイオンの大半は高い求核性を持ち、空気によって素早く酸化される[1]。
セレノール類の沸点は、チオールの沸点よりもわずかに大きい傾向にある。これはファンデルワールス結合の重要性が原子が大きくなるほど強くなるためである。揮発性セレノール類は、ひどい悪臭を示す。
応用および存在
[編集]セレノール類は、セレンの高い毒性とSe-H結合の弱さのため、商業的な応用はほとんどされていない。セレノール類の共役塩基であるセレノラート類は有機合成において限定的な応用が成されている。

生化学的役割
[編集]セレノール類は特定の生物学的過程において重要である。ほ乳類では活性部位にセレノール類を含む3種類の酵素(グルタチオンペルオキシダーゼ、ヨードチオニンデヨージナーゼ、チオレドキシンジスルフィドレダクターゼ)が発見されている。これらのタンパク質中のセレノール類は必須アミノ酸であるセレノシステインの一部分である[1]。セレノール類は還元剤として機能し、セレネン酸誘導体(RSe-OH)となり、セレネン酸は次にチオール含有酵素によって再び還元される。メタンセレノール(一般名メチルセレノール、CH3SeH)は、セレノメチオニンと細菌メチオニンγ-リアーゼ(METase)とのin vitroでのインキュベーションや、セレニドイオンの生物学的メチル化や、メタンセレニン酸((CH3SeO2H))のin vivoでの還元によって生産できる。メタンセレノールによって特定の有機セレン化合物の抗がん活性が説明できるかもしれない[2][3][4]。メタンセレノールの前駆体は、がん予防およびがん治療において活発に研究されている。これらの研究において、んメタンセレノールがエタンセレノールや2-プロパンセレノールよりも高い生物活性を示すことが明らかにされている[5]。
調製
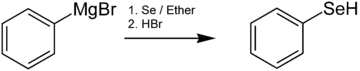
[編集]セレノール類は通常、有機リチウム試薬あるいはグリニャール試薬とSe元素との反応によって調製される。例えば、ベンゼンセレノールはフェニルマグネシウムブロミドとセレンを反応させた後に酸性にすることによって生成される[6]。
その他の調製法としては、セレノ尿素のアルキル化とその後の加水分解がある。セレノール類はジセレニドの還元と得られたセレノアートのプロトン化によってしばしば作られる。
- 2 RSeSeR + 2 LiHB(C2H5)3 → 2 RSeLi + 2 B(C2H5)3 + H2
- RSeLi + HCl → RSeH + LiCl
ジメチルジセレニドは細胞内のメタンセレノールによって容易に還元できる[7]。
反応
[編集]セレノール類は、Se-Se結合を含む化合物であるジセレニド類に容易に酸化される。例えば、ベンゼンセレノールを臭素で処理するとジフェニルジセレニドが得られる。
- 2 C6H5SeH + Br2 → (C6H5Se)2 + 2 HBr
安全性
[編集]有機セレン化合物(あるいはセレン化合物)は、微量のSeが健康のために必要であるにもかかわらず、蓄積される毒物である。
脚注
[編集]- ^ a b Wessjohann, L.A.; Schneider, A.; Abbas, M.; Brandt, W. (2007). “Selenium in Chemistry and Biochemistry in Comparison to Sulfur”. Biol. Chem. 388: 997–1006. doi:10.1515/BC.2007.138.
- ^ Zeng, H.; Briske-Anderson, M.; Wu, M.; Moyer, M. P. (2012). “Methylselenol, a selenium metabolite, plays common and different roles in cancerous colon HCT116 cell and noncancerous NCM460 colon cell proliferation”. Nutrition and Cancer 64: 128–135. doi:10.1080/01635581.2012.630555.
- ^ Fernandes, A.P.; Wallenberg, M.; Gandin, V.; Misra1, S.; Marzano, C.; Rigobello, M. P.; Kumar, S.; Björnstedt, M. (2012). “Methylselenol formed by spontaneous methylation of selenide is a superior selenium substrate to the thioredoxin and glutaredoxin systems”. PLoS ONE 7: e50727. doi:10.1371/journal.pone.0050727.
- ^ Ip, C.; Dong, Y.; Ganther, H. E. (2002). “New concepts in selenium chemoprevention”. Cancer Metastasis Rev. 21: 281–289.
- ^ Zuazo, A.; Plano, D.; Ansó, E.; Lizarraga, E.; Font, M.; Irujo, J.J.M. (2012). “Cytotoxic and proapototic activities of imidoselenocarbamate derivatives are dependent on the release of methylselenol”. Chem. Res. Toxicol. 25: 2479–2489. doi:10.1021/tx300306t.
- ^ Foster, D. G. (1955). "Selenophenol". Organic Syntheses (英語).; Collective Volume, vol. 3, p. 771
- ^ Gabel-Jensen, C.; Lunoe, K.; Gammelgaard, B. (2010). “Formation of methylselenol, dimethylselenide and dimethyldiselenide in in vitro metabolism models determined by headspace GC-MS”. Metallomics 2: 167–173.