프로게스테론 (약품)
| |

| |
| 체계적 명칭 (IUPAC 명명법) | |
|---|---|
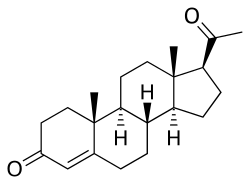
| (8S,9S,10R,13S,14S,17S)-17-acetyl-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one | |
| 식별 정보 | |
| CAS 등록번호 | 57-83-0 |
| ATC 코드 | G03DA04 |
| PubChem | 5994 |
| 드러그뱅크 | DB00396 |
| ChemSpider | 5773 |
| 화학적 성질 | |
| 화학식 | C21H30O2 |
| 분자량 | ? |
| 유의어 | P4; Pregnenedione; Pregn-4-ene-3,20-dione[1] |
| 물리적 성질 | |
| 녹는점 | 126 °C (259 °F) |
| 비선광도 | [α]D25 = +172 to +182° (2% in dioxane, β-form) |
| 약동학 정보 | |
| 생체적합성 | Oral: <2.4%[2] Vaginal (micronized insert): 4–8%[3][4][5] |
| 단백질 결합 | 98–99%:[6][7] • Albumin: 80% • CBG: 18% • SHBG: <1% • Free: 1–2% |
| 동등생물의약품 | ? |
| 약물 대사 | Mainly liver: • 5α- and 5β-reductase • 3α- and 3β-HSD • 20α- and 20β-HSD • Conjugation • 17α-Hydroxylase • 21-Hydroxylase • CYPs (e.g., CYP3A4) |
| 생물학적 반감기 | • Oral: 5 hours (with food)[8] * Sublingual: 6–7 hours[9] • Vaginal: 14–50 hours[10][9] • Topical: 30–40 hours[11] • IM: 20–28 hours[12][10][13] • SC: 13–18 hours[13] • IV: 3–90 minutes[14] |
| 배출 | Bile and urine[15][16] |
| 처방 주의사항 | |
| 임부투여안전성 | ? |
| 법적 상태 | |
| 투여 방법 | By mouth, sublingual, topical, vaginal, rectal, intramuscular, subcutaneous, intrauterine |
프로게스테론(progesterone, P4)은 Prometrium 등의 브랜드 이름으로 판매되는 약품이며 천연적으로 발생하는 스테로이드 호르몬이다.[20]
같이 보기
[편집]- 에스트로겐(estrogen)
각주
[편집]- ↑ Adler N, Pfaff D, Goy RW (2012년 12월 6일). 《Handbook of Behavioral Neurobiology Volume 7 Reproduction》 1판. New York: Plenum Press. 189쪽. ISBN 978-1-4684-4834-4. 2015년 7월 4일에 확인함.
- ↑ Levine H, Watson N (March 2000). “Comparison of the pharmacokinetics of crinone 8% administered vaginally versus Prometrium administered orally in postmenopausal women(3)”. 《Fertility and Sterility》 73 (3): 516–521. doi:10.1016/S0015-0282(99)00553-1. PMID 10689005.
- ↑ Griesinger G, Tournaye H, Macklon N, Petraglia F, Arck P, Blockeel C, van Amsterdam P, Pexman-Fieth C, Fauser BC (February 2019). “Dydrogesterone: pharmacological profile and mechanism of action as luteal phase support in assisted reproduction”. 《Reproductive Biomedicine Online》 38 (2): 249–259. doi:10.1016/j.rbmo.2018.11.017. PMID 30595525.
- ↑ Pandya MR, Gopeenathan P, Gopinath PM, Das SK, Sauhta M, Shinde V (2016). “Evaluating the clinical efficacy and safety of progestogens in the management of threatened and recurrent miscarriage in early pregnancy-A review of the literature”. 《Indian Journal of Obstetrics and Gynecology Research》 3 (2): 157. doi:10.5958/2394-2754.2016.00043.6. ISSN 2394-2746. S2CID 36586762.
- ↑ Paulson RJ, Collins MG, Yankov VI (November 2014). “Progesterone pharmacokinetics and pharmacodynamics with 3 dosages and 2 regimens of an effervescent micronized progesterone vaginal insert”. 《The Journal of Clinical Endocrinology and Metabolism》 99 (11): 4241–4249. doi:10.1210/jc.2013-3937. PMID 24606090.
- ↑ Fritz MA, Speroff L (2012년 3월 28일). 《Clinical Gynecologic Endocrinology and Infertility》. Lippincott Williams & Wilkins. 44–쪽. ISBN 978-1-4511-4847-3.
- ↑ Marshall WJ, Bangert SK (2008). 《Clinical Chemistry》. Elsevier Health Sciences. 192–쪽. ISBN 978-0-7234-3455-9.
- ↑ Pickar JH, Bon C, Amadio JM, Mirkin S, Bernick B (December 2015). “Pharmacokinetics of the first combination 17β-estradiol/progesterone capsule in clinical development for menopausal hormone therapy”. 《Menopause》 22 (12): 1308–1316. doi:10.1097/GME.0000000000000467. PMC 4666011. PMID 25944519.
- ↑ 가 나 Khomyak NV, Mamchur VI, Khomyak EV (2014). “Клинико-фармакологические особенности современных лекарственных форм микронизированного прогестерона, применяющихся во время беременности.” [Clinical and pharmacological features of modern dosage forms of micronized progesterone used during pregnancy.] (PDF). 《Доровье》 4: 90.
- ↑ 가 나 “Crinone® 4% and Crinone® 8% (progesterone gel)” (PDF). 《Watson Pharma, Inc.》. U.S. Food and Drug Administration. August 2013.
- ↑ Mircioiu C, Perju A, Griu E, Calin G, Neagu A, Enachescu D, Miron DS (1998). “Pharmacokinetics of progesterone in postmenopausal women: 2. Pharmacokinetics following percutaneous administration”. 《European Journal of Drug Metabolism and Pharmacokinetics》 23 (3): 397–402. doi:10.1007/BF03192300. PMID 9842983. S2CID 32772029.
- ↑ Simon JA, Robinson DE, Andrews MC, Hildebrand JR, Rocci ML, Blake RE, Hodgen GD (July 1993). “The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone”. 《Fertility and Sterility》 60 (1): 26–33. doi:10.1016/S0015-0282(16)56031-2. PMID 8513955.
- ↑ 가 나 Cometti B (November 2015). “Pharmaceutical and clinical development of a novel progesterone formulation”. 《Acta Obstetricia et Gynecologica Scandinavica》 94 (Suppl 161): 28–37. doi:10.1111/aogs.12765. PMID 26342177. S2CID 31974637.
The administration of progesterone in injectable or vaginal form is more efficient than by the oral route, since it avoids the metabolic losses of progesterone encountered with oral administration resulting from the hepatic first-pass effect (32). In addition, the injectable forms avoid the need for higher doses that cause a fairly large number of side-effects, such as somnolence, sedation, anxiety, irritability and depression (33).
- ↑ Aufrère MB, Benson H (June 1976). “Progesterone: an overview and recent advances”. 《Journal of Pharmaceutical Sciences》 65 (6): 783–800. doi:10.1002/jps.2600650602. PMID 945344.
- ↑ “Prometrium (progesterone, USP) Capsules 100 mg” (PDF). 《Solvay Pharmaceuticals, Inc.》. U.S. Food and Drug Administration. 1998.
- ↑ “Progesterone Injection USP in Sesame Oil for Intramuscular Use Only Rx Only” (PDF). U.S. Food and Drug Administration. January 2007.
- ↑ “Regulatory Decision Summary for pms-Progesterone”. 《Drug and Health Product Register》. 2014년 10월 23일.
- ↑ “Reproductive health”. 《Health Canada》. 2018년 5월 9일. 2024년 4월 13일에 확인함.
- ↑ “Health product highlights 2021: Annexes of products approved in 2021”. 《Health Canada》. 2022년 8월 3일. 2024년 3월 25일에 확인함.
- ↑ Kuhl H (August 2005). “Pharmacology of estrogens and progestogens: influence of different routes of administration”. 《Climacteric》 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.