PBT2
| |
| Nazivi | |
|---|---|
| IUPAC naziv
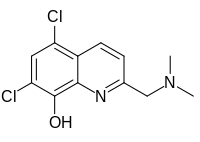
5,7-Dihloro-2-[(dimetilamino)metil]hinolin-8-ol
| |
| Identifikacija | |
3D model (Jmol)
|
|
| ChemSpider | |
| UNII | |
| |
| Svojstva | |
| C12H12Cl2N2O | |
| Molarna masa | 271,14 g·mol−1 |
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |
| Reference infokutije | |
PBT2 je ekperimentalni kandidat leka. On je druga generacija 8-hidroksihinolinskog analoga[3] koji je trebalo bude zamena za kliohinol i potencijalni tretman za Alchajmerovu bolest.[4]
Klinička ispitivanja
[уреди | уреди извор]PBT2 je bio u fazi II kliničkih ispitivanja za Alchajmerovu bolest i Hantingtonovu bolest. Rezultati za Alchajmerovu bolest nisu bili uspešni,[5][6] i ne postoji evidencija da je PBT2 koristan za tretiranje Alchajmerove demencije.[7] Rezultati ispitivanja za Hantingtonovu bolest takođe nisu bili pozitivni.[8][9]
Reference
[уреди | уреди извор]- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
- ^ Adlard, Paul A.; Cherny, Robert A.; Finkelstein, David I.; Gautier, Elisabeth; Robb, Elysia; Cortes, Mikhalina; Volitakis, Irene; Liu, Xiang; et al. (2008). „Rapid Restoration of Cognition in Alzheimer's Transgenic Mice with 8-Hydroxy Quinoline Analogs is Associated with Decreased Interstitial Aβ”. Neuron. 59 (1): 43—55. PMID 18614028. doi:10.1016/j.neuron.2008.06.018.
- ^ Duce, James A.; Tsatsanis, Andrew; Cater, Michael A.; James, Simon A.; Robb, Elysia; Wikhe, Krutika; Leong, Su Ling; Perez, Keyla; et al. (2010). „Iron-Export Ferroxidase Activity of β-Amyloid Precursor Protein is Inhibited by Zinc in Alzheimer's Disease”. Cell. 142 (6): 857—67. PMC 2943017
 . PMID 20817278. doi:10.1016/j.cell.2010.08.014. Генерални сажетак – ScienceDaily (2010-09-10).
. PMID 20817278. doi:10.1016/j.cell.2010.08.014. Генерални сажетак – ScienceDaily (2010-09-10).
- ^ „Prana Biotech Plunges 76%; Drug Fails in Alzheimer Study”. Bloomberg News. 2014-03-31.
- ^ „PBT2 Takes a Dive in Phase 2 Alzheimer's Trial”. 2014.
- ^ „There is no evidence that MPACs (PBT1 or PBT2) are of benefit in Alzheimer's dementia”. Cochrane Database of Systematic Reviews: Plain Language Summaries. PubMed Health.
- ^ „Prana Bio Huntington's Disease Drug Fails Key Efficacy Hurdles”. thestreet.com.
- ^ Huntington Study Group Reach2HD Investigators (2015). „Safety, tolerability, and efficacy of PBT2 in Huntington's disease: A phase 2, randomised, double-blind, placebo-controlled trial”. The Lancet Neurology. 14 (1): 39—47. PMID 25467848. doi:10.1016/S1474-4422(14)70262-5.