白僵菌素
| 白僵菌素 | |
|---|---|
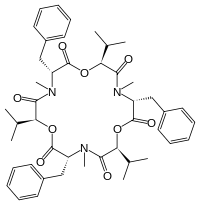
| |

| |
| IUPAC名 (3S,6R,9S,12R,15S,18R)-3,9,15-Tribenzyl-6,12,18-triisopropyl-4,10,16-trimethyl-1,7,13-trioxa-4,10,16-triazacyclooctadecane-2,5,8,11,14,17-hexone | |
| 别名 | 白僵菌毒素 |
| 识别 | |
| CAS号 | 26048-05-5 |
| PubChem | 3007984 |
| ChemSpider | 2277520 |
| SMILES |
|
| InChI |
|
| InChIKey | GYSCAQFHASJXRS-FFCOJMSVBB |
| ChEBI | 3000 |
| KEGG | C11590 |
| 性质 | |
| 化学式 | C45H57N3O9 |
| 摩尔质量 | 783.95 g·mol−1 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
白僵菌素(英語:Beauvericin)是一种具有抗生素和杀虫剂性质的酯肽(缩酚酸肽),隶属于恩镰孢菌素家族。其最早从球孢白僵菌(Beauveria bassiana)中分离得到,包括几种镰孢菌在内的其他真菌也会产生白僵菌素[1][2]。其一般出现在受这些真菌污染的谷物中,如玉米、小麦、大麦等[2][3][4]。白僵菌素对革兰氏阳性菌和分枝杆菌有抑制活性,也能诱导哺乳动物细胞的程序性死亡[2]。
从化学结构上看,白僵菌素是一种环状六酯肽,由N-甲基-苯丙氨酰基和D-羟基-异戊酰基交替连接得到。其可与金属离子配位,可与碱金属和碱土金属离子螯合并透过细胞膜[來源請求]。
白僵菌素与抗真菌药物酮康唑联合使用(剂量为 0.1 μg/ml)对近平滑念珠菌(Candida parapsilosis)具有体外杀菌作用。在小鼠模型中也显示出提高存活率和低毒性的特性[5]。
参考文献
[编辑]- ^ Hamill RL, Higgens CE, Boaz HE, Gorman M. The structure of beauvericin, a new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Letters. 1969, 10 (49): 4255–4258. doi:10.1016/S0040-4039(01)88668-8.
- ^ 2.0 2.1 2.2 Logrieco A, Moretti A, Castella G, et al. Beauvericin Production by Fusarium Species. Appl Environ Microbiol. 1998, 64 (8): 3084–8. Bibcode:1998ApEnM..64.3084L. PMC 106821
 . PMID 9687479. doi:10.1128/AEM.64.8.3084-3088.1998.
. PMID 9687479. doi:10.1128/AEM.64.8.3084-3088.1998.
- ^ Logrieco A, Rizzo A, Ferracane R, Ritieni A. Occurrence of Beauvericin and Enniatins in Wheat Affected by Fusarium avenaceum Head Blight. Appl Environ Microbiol. 2002, 68 (1): 82–5. Bibcode:2002ApEnM..68...82L. PMC 126553
 . PMID 11772612. doi:10.1128/AEM.68.1.82-85.2002.
. PMID 11772612. doi:10.1128/AEM.68.1.82-85.2002.
- ^ Jestoi M, Rokka M, Yli-Mattila T, Parikka P, Rizzo A, Peltonen K. Presence and concentrations of the Fusarium-related mycotoxins beauvericin, enniatins and moniliformin in finnish grain samples. Food Additives and Contaminants. 2004, 21 (8): 794–802. PMID 15370831. S2CID 19565366. doi:10.1080/02652030410001713906.
- ^ Zhang L; Yan K; Zhang Y; Huang R; Bian J; Zheng C; Sun H; Chen Z; Sun N; An R; Min F; Zhao W; Zhuo Y; You J; Song Y; Yu Z; Liu Z; Yang K; Gao H; Dai H; Zhang X; Wang J; Fu C; Pei G; Liu J; Zhang S; Goodfellow M; Jiang Y; Kuai J; Zhou G; Chen X.K. High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. Proc Natl Acad Sci U S A. 2007, 104 (11): 4606–11. Bibcode:2007PNAS..104.4606Z. PMC 1838648
 . PMID 17360571. doi:10.1073/pnas.0609370104
. PMID 17360571. doi:10.1073/pnas.0609370104  .
.