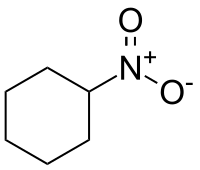
硝基环己烷
| 硝基环己烷 | |
|---|---|
| |

| |
| IUPAC名 Nitrocyclohexane | |
| 识别 | |
| CAS号 | 1122-60-7 |
| PubChem | 14285 |
| ChemSpider | 13647 |
| SMILES |
|
| InChI |
|
| InChIKey | NJNQUTDUIPVROZ-UHFFFAOYAA |
| 性质 | |
| 化学式 | C6H11NO2 |
| 摩尔质量 | 129.16 g·mol−1 |
| 密度 | 1.061 g/cm3 |
| 熔点 | -34 °C(239 K) |
| 沸点 | 205.8 °C(479 K) |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
硝基环己烷可由二氧化氮(或硝酸)和环己烷反应制得。[1][2]次氟酸-乙腈氧化叠氮环己烷或环己胺也能得到硝基环己烷。[3]它可以被硼氢化钠还原为环己胺。[4]它和甲醛反应,可以得到1-硝基-1-羟甲基环己烷。[5]它在碱性条件下和高锰酸钾反应,可以得到环己酮。[6]
参考文献
[编辑]- ^ Ishii, Yasutaka; Nakano, Tatsuya. Process for the preparation of nitro compounds and method for the removal of nitrogen dioxide. 2000 WO 2000069803 A1.
- ^ Shinji Isozaki, Yoshiki Nishiwaki, Satoshi Sakaguchi, Yasutaka Ishii. Nitration of alkanes with nitric acid catalyzed by N-hydroxyphthalimide. Chemical Communications. 2001, (15): 1352–1353 [2021-12-10]. doi:10.1039/b102374h.
- ^ Christopher B. McPake, Christopher B. Murray, Graham Sandford. Sequential Continuous Flow Processes for the Oxidation of Amines and Azides by using HOF⋅MeCN. ChemSusChem. 2012-02-13, 5 (2): 312–319 [2021-12-10]. doi:10.1002/cssc.201100423 (英语).
- ^ Wei-Guo Jia, Mei Li, Xue-Ting Zhi, Li-Li Gao, Jun Du. Copper(II) complex with oxazoline ligand: Synthesis, structures and catalytic activity for nitro compounds reduction. Journal of Molecular Structure. 2020-10, 1217: 128349 [2021-12-10]. doi:10.1016/j.molstruc.2020.128349. (原始内容存档于2020-05-09) (英语).
- ^ William B. Wheatley. α,α-Dimethylcholine: Esters and Carbamates 1. Journal of the American Chemical Society. 1954-05, 76 (10): 2832–2835 [2021-12-10]. ISSN 0002-7863. doi:10.1021/ja01639a068. (原始内容存档于2021-12-04) (英语).
- ^ Jörg Sedelmeier, Steven V. Ley, Ian R. Baxendale, Marcus Baumann. KMnO 4 -Mediated Oxidation as a Continuous Flow Process. Organic Letters. 2010-08-20, 12 (16): 3618–3621 [2021-12-10]. ISSN 1523-7060. doi:10.1021/ol101345z. (原始内容存档于2021-12-04) (英语).