Galopamil
|
| (IUPAC) ime
|
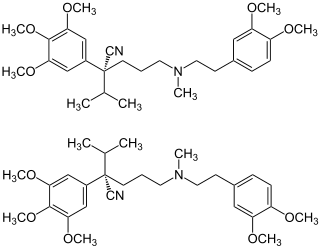
| (RS)-5-[2-(3,4-Dimetoksifenil)etil-metilamino]-2-propan-2-il-2-(3,4,5-trimetoksifenil)pentan nitril
|
| Klinički podaci
|
| AHFS/Drugs.com
|
Internacionalno ime leka
|
| Identifikatori
|
| CAS broj
|
16662-46-7
|
| ATC kod
|
C08DA02
|
| PubChem[1][2]
|
1234
|
| ChemSpider[3]
|
1197
|
| UNII
|
39WPC8JHR8  Y Y
|
| KEGG[4]
|
D01969  Y Y
|
| ChEMBL[5]
|
CHEMBL51149  Y Y
|
| Hemijski podaci
|
| Formula
|
C28H40N2O5
|
| Mol. masa
|
484,62 g/mol
|
| SMILES
|
eMolekuli & PubHem
|
| InChI |
|---|
InChI=1S/C28H40N2O5/c1-20(2)28(19-29,22-17-25(33-6)27(35-8)26(18-22)34-7)13-9-14-30(3)15-12-21-10-11-23(31-4)24(16-21)32-5/h10-11,16-18,20H,9,12-15H2,1-8H3  N N
Key: XQLWNAFCTODIRK-UHFFFAOYSA-N  N N |
|
| Farmakoinformacioni podaci
|
| Trudnoća
|
?
|
| Pravni status
|
|
Galopamil (INN) je L-tip blokatora kalcijumovog kanala. On je je analog verapamila. Galopamil se koristi za lečenje srčanih aritmija.[6][7]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846. edit
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594. edit
- ↑ Sewing K-F, Hannemann H (1983). „Calcium Channel Antagonists Verapamil and Gallopamil Are Powerful Inhibitors of Acid Secretion in Isolated and Enriched Guinea Pig Parietal Cells”. Pharmacology 27: 9–14. DOI:10.1159/000137824.
- ↑ Mitić R., Biševac B., Stanojević Z., Bursać M., Đokić T. (2009). Efekt verapamila na trahealni odgovor izazvan histaminom i acetilholinom. 37. pp. 1-9. Arhivirano iz originala na datum 2011-05-31. Pristupljeno 2014-04-05.
