Almoreksant
| |||
| (IUPAC) ime | |||
|---|---|---|---|
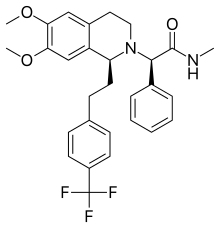
| (2R)-2-[(1S)- 6,7-dimethoxy- 12-[4-(trifluoromethyl)phenyl]ethyl 3,4-dihydroisoquinolin-2(1H)-yl]- N-methyl- 2-phenylacetamide | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 871224-64-5 | ||
| ATC kod | nije dodeljen | ||
| PubChem[1][2] | 23727689 | ||
| ChemSpider[3] | 21377865 | ||
| Hemijski podaci | |||
| Formula | C29H32ClF3N2O3 | ||
| Mol. masa | 512,6 g/mol (slobodna baza) | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Metabolizam | Hepatčki | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | |||
| Način primene | Oralno | ||
Almoreksant (INN), kode ACT-078573, je kompetitivni antagonist OX1 i OX2 oreksinskih receptor. Ovaj lek je razvila farmaceutska kompanija Actelion za lečenje primarne insomnije. Njegova klinička ispitivanja faze III su završen su novembra 2009.[4]
Januara 2011 Actelion i GSK su objavili prestanak daljih kliničkih ispitivanja zbog nuspojava.[5]
Almoreksant je kompetitivni, dualni antagonist receptora OX1 i OX2. On selektivno inhibira aktivaciju OX1 i OX2 receptora.
Almoreksant je bio u razvoju za lečenje nesanice.
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ „Almorexant in Adult Subjects With Chronic Primary Insomnia (RESTORA 1), ClinicalTrials.gov”. Pristupljeno 3. 02. 2010.
- ↑ „Actelion and GSK Discontinue Clinical Development of Almorexant”..