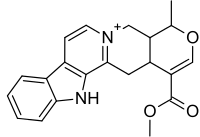
Alstonin
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| (19α,20α)-16-(Metoksikarbonil)-19-metil-3,4,5,6,16,17-heksadehidro-18-oksajohimban-4-ium | |||
| Klinički podaci | |||
| Identifikatori | |||
| ATC kod | nije dodeljen | ||
| PubChem[1][2] | 441979 | ||
| ChemSpider[3] | 149308 | ||
| Hemijski podaci | |||
| Formula | C21H21N2O3 | ||
| Mol. masa | 349,402496 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | |||
Alstonin je indolni alkaloid i mogući antipsihotički sastojak raznih biljnih vrsta, uključujući Alstonia boonei, Catharanthus roseus, Picralima nitida, Rauwolfia caffra i Rauwolfia vomitoria.[4] U prikliničkim ispitivanjima je pokazano da alstonin ublažuje hiperlokomociju indukovanu dejstvom MK-801, kao i deficit radne memorije i društveno povlačenje.[5] U prikliničkim studijama je takođe pokazano da on ispoljava dejstva slična anksioliticima.[4] On umanjuje amfetaminom indukovanu letalnost i stereotipnost, kao i apomorfinom indukovanu stereotipnost.[4] On umanjuje haloperidolom indukovanu katalepsiju.[6] Smatra se da su ta dejstva posredovana stimulacijom 5-HT2C receptora.[7] On takođe, slično klozapinu, inhibira ponovno preuzimanje glutamata u hipokampalnim režnjevima.[8] Za razliku od klozapina, on ne manifestuje prokonvulzivno dejstvo kod miševa.[9]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ 4,0 4,1 4,2 Elisabetsky, E; Costa-Campos, L (March 2006). „The Alkaloid Alstonine: A Review of Its Pharmacological Properties” (PDF). Evidence-based Complementary and Alternative Medicine 3 (1): 39-48. DOI:10.1093/ecam/nek011. PMC 1375234. PMID 16550222.
- ↑ Linck, VM; Bessa, MM; Herrmann, AP; Iwu, MM; Okunji, CO; Elisabetsky, E (January 2012). „5-HT2A/C receptors mediate the antipsychotic-like effects of alstonine”. Progress in Neuro-Psychopharmacology and Biological Psychiatry 36 (1): 29-33. DOI:10.1016/j.pnpbp.2011.08.022. PMID 21925231.
- ↑ Linck, VM; Herrmann, AP; Piato, LN; Detanico, BC; Figueir, M; Flório, J; Iwu, MM; Okunji, CO; Leal, MB; Elisabetsky, E (July 2011). „Alstonine as an Antipsychotic: Effects on Brain Amines and Metabolic Changes” (PDF). Evidence-Based Complementary and Alternative Medicine 2011 (418597). DOI:10.1093/ecam/nep002.
- ↑ Gross, G; Geyer, MA, ur. (2012). Current Antipsychotics. Handbook of Experimental Pharmacology. 212. Springer Berlin Heidelberg. DOI:10.1007/978-3-642-25761-2. ISBN 978-3-642-25761-2. ISSN 1865-0325.
- ↑ Herrmann, AP; Lunardi, P; Pilz, LK; Tramontina, AC; Linck, VM; Okunji, CO; Gonçalves, CA; Elisabetsky, E (December 2012). „Effects of the putative antipsychotic alstonine on glutamate uptake in acute hippocampal slices”. Neurochemistry International 61 (7): 1144-1150. DOI:10.1016/j.neuint.2012.08.006. PMID 22940693.
- ↑ Costa-Campos L, Iwu M, Elisabetsky E (August 2004). „Lack of pro-convulsant activity of the antipsychotic alkaloid alstonine”. Journal of Ethnopharmacology 93 (2-3): 307-310. DOI:10.1016/j.jep.2004.03.056. PMID 15234769.