Tolazolin
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| 2-benzil-4,5-dihidro-1H-imidazol | |||
| Klinički podaci | |||
| AHFS/Drugs.com | Internacionalno ime leka | ||
| Identifikatori | |||
| CAS broj | 59-98-3 | ||
| ATC kod | C04AB02 M02AX02 QV03 | ||
| PubChem[1][2] | 5504 | ||
| DrugBank | DB00797 | ||
| ChemSpider[3] | 5303 | ||
| UNII | CHH9H12AQ3 | ||
| KEGG[4] | D08614 | ||
| ChEBI | CHEBI:28502 | ||
| ChEMBL[5] | CHEMBL770 | ||
| Hemijski podaci | |||
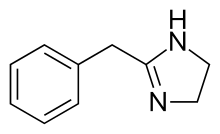
| Formula | C10H12N2 | ||
| Mol. masa | 160,216 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | |||
| Način primene | IV | ||
Tolazolin je neselektivni kompetitivni antagonist α-adrenergičkog receptora. On je vazodilatator koji se koristi za tretiranje spazama perifernih krvnih sudova (kao u akrocijanozi). On je takođe uspešno korišćen kao antidot za poništavanje jake periferne vazokonstrikcije koja se može javiti usled predoziranja pojedinih agonista 5-HT2A receptora kao što su LSD, DOB i Bromo-DragonFLY, koji mogu da dovedu do gangrene ako se ne tretiraju.[6][7]
On se najčešće koristi u veterini, za poništavanje ksilazinom indukovane sedacije.[8][9]
Tolazolin, 2-benzil-2-imidazolin, se može sintetisati putem heterociklizacije ethil ester iminofenzilsirćetne kiseline sa etilen diaminom. Struktura tolazolina je veoma slična sa α-adrenergičkim agonistima, koji su antiedemski simpatomimetici.[10][11][12][13]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Bowen JS, Davis GB, Kearney TE, Bardin J. Diffuse vascular spasm associated with 4-bromo-2,5-dimethoxyamphetamine ingestion. Journal of the American Medical Association 1983 Mar 18;249(11):1477-9. PMID 6827726
- ↑ Thorlacius K, Borna C, Personne M. Bromo-dragon fly--life-threatening drug. Can cause tissue necrosis as demonstrated by the first described case. (Swedish) Lakartidningen. 2008 Apr 16-22;105(16):1199-200. PMID 18522262
- ↑ Boothe DM (2001). „Anticonvulsant drugs and analeptic agents”. u: Adams HR (ed.). Veterinary pharmacology and therapeutics. Ames: Iowa State University Press. str. 378–9. ISBN 0-8138-1743-9. Retrieved September 8, 2008.
- ↑ Hall LW, Clarke KW, Trim CM (2001). „Principles of sedation, analgesia and premedication”. Veterinary anaesthesia. Philadelphia: W.B. Saunders. str. 90–1. ISBN 0-7020-2035-4.
- ↑ A. Sonn, U.S. Patent 2.161.938 (1939).
- ↑ W. Wustrow, P. Herold, DE 615527 (1934).
- ↑ M. Hartmann, H.O. Isler, DE 687196 (1938).
- ↑ N. Guenter, DE 3043562 (1982).
- Boothe DM (2001). „Anticonvulsant drugs and analeptic agents”. u: Adams HR (ed.). Veterinary pharmacology and therapeutics. Ames: Iowa State University Press. str. 378–9. ISBN 0-8138-1743-9. Retrieved September 8, 2008 .
- Hall LW, Clarke KW, Trim CM (2001). „Principles of sedation, analgesia and premedication”. Veterinary anaesthesia. Philadelphia: W.B. Saunders. str. 90–1. ISBN 0-7020-2035-4.
